What is scDiabody
scDiabody is a type of bispecific antibody (BsAb) that can simultaneously recognize two different antigens. BsAb is a class of biologics with broad applications in tumor immunotherapy, diagnosis, and radiotherapy. scDiabody is a common format of BsAb, composed of two single-chain Fv (scFv) fragments connected by a linker peptide. Each scFv fragment consists of a variable heavy chain (VH) and a variable light chain (VL). scDiabody offers unique advantages, including high affinity, low immunogenicity, easy expression, and purification.
Structural Characteristics of scDiabody
The basic structure of scDiabody consists of two identical or different scFv chains, each containing a VH and a VL domain, connected by a short linker peptide. Due to the limited length of the linker peptide, the two scFv chains form a dimer in a cross-over manner, resulting in two antigen binding sites. This structure provides scDiabody with high affinity and stability, while reducing immunogenicity and aggregation tendency. Depending on the combination and number of scFv chains, scDiabody can have various forms. Bifunctional scDiabody, the most common form, consists of two different scFv chains, and can recognize two different antigens simultaneously. Monofunctional scDiabody comprises of two identical scFv chains and can only recognize one antigen. Bivalent scDiabody consists of four identical or different scFv chains and enhances antigen binding and cross-linking ability. The structure of scDiabody is influenced by factors such as linker peptide length, VH and VL arrangement order, and linker peptide sequence, etc. Generally speaking, the shorter the linker peptide length, the easier the dimer formation. The VH and VL arrangement order determines the relative position and direction of the antigen binding sites, and the linker peptide sequence affects the stability and solubility of the dimer. These factors need to be considered when designing and optimizing scDiabody, these factors need to be considered for their impact on its structure and function.
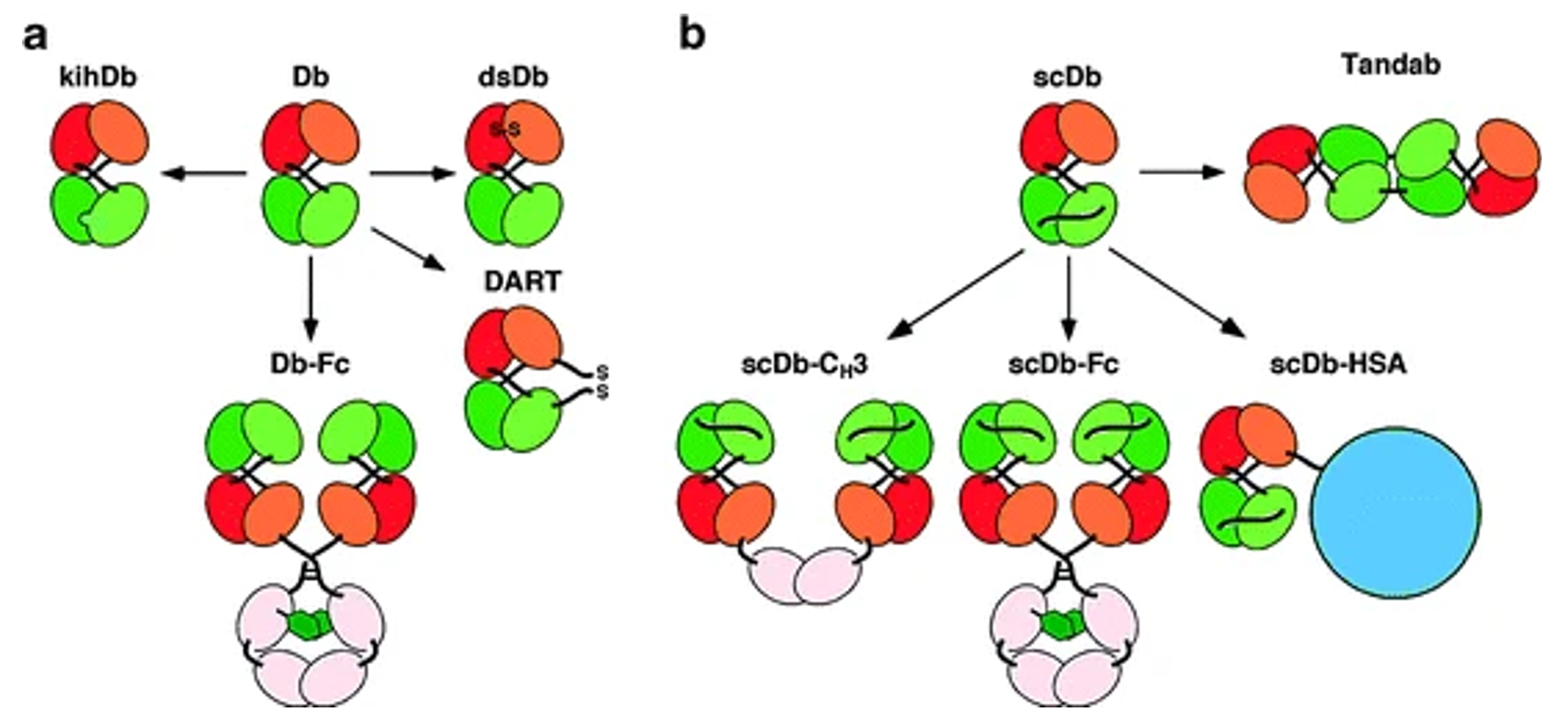
Fig.1 Derivatives of Bispecific Diabodies and Single-Chain Diabodies (Dafne Müller, 2011)
Clinical Application of scDiabody
As a bispecific antibody (BsAb), scDiabody has the capability to recognize two different antigens simultaneously, enabling various therapeutic effects, such as redirecting T cells to kill tumor cells, blocking two signaling pathways, and enhancing antigen presentation. Therefore, scDiabody holds great potential in clinical applications, particularly in the field of tumor immunotherapy.
Currently, two scDiabody drugs targeting CD19/CD3 have received marketing approval: Blinatumomab and Glofitamab. Blinatumomab is a bifunctional scDiabody composed of two different scFv fragments connected together, which can simultaneously recognize CD19 on B cells and CD3 on T cells, redirecting T cells to B cells and inducing T cell-mediated B cell killing. It was approved in the European Union in 2014 and in the United States in 2017 for the treatment of relapsed or refractory acute lymphoblastic leukemia (R/R ALL), and was approved in the United States in 2018 for the treatment of CD19-positive B-cell precursor acute lymphoblastic leukemia (BCP-ALL). Glofitamab, a monofunctional scDiabody, composed of two identical scFv fragments connected together, which can recognize CD19 and bind to CD3 through the Fc region, thereby redirecting T cells to CD19-positive tumor cells and inducing T cell-mediated tumor cell killing. It was approved for the treatment of relapsed or refractory non-Hodgkin lymphoma (R/R NHL) in the European Union in 2020.
Several scDiabody drugs targeting different antigens are currently in clinical trials (Table 1). Chinese companies and research institutions primarily develop drugs targeting GPC3/CD3, EGFR/CD3, and other targets for treating malignant tumors like liver cancer and lung cancer. American or European companies and research institutions focus on targets such as PSMA/CD3, CEA/CD3, mainly for prostate and colon cancer.
Table 1. scDiabody Drugs in Clinical Trials
|
scDiabody drug
|
Target
|
Clinical trial phase
|
Main indication
|
Main developer
|
|
KN046
|
PD-L1/CD3
|
I/II
|
Lung cancer, esophageal cancer, etc.
|
Alphamab
|
|
KN026
|
HER2/CD3
|
I/II
|
Breast cancer, gastric cancer, etc.
|
Alphamab
|
|
KN019
|
GPC3/CD3
|
I
|
Liver cancer, ovarian cancer, etc.
|
Alphamab
|
|
KN046
|
EGFR/CD3
|
I
|
Lung cancer, colorectal cancer, etc.
|
Alphamab
|
|
ERY974
|
GPC3/CD3
|
I
|
Liver cancer, lung cancer, etc.
|
Chugai/Roche
|
|
REGN4018
|
MUC16/CD3
|
I
|
Ovarian cancer, pancreatic cancer, etc.
|
Regeneron
|
|
AMG 596
|
EGFRvIII/CD3
|
I
|
Glioblastoma multiforme, etc.
|
Amgen
|
|
AMG 673
|
CD33/CD3
|
I
|
Acute myeloid leukemia, etc.
|
Amgen
|
|
AMG 701
|
BCMA/CD3
|
I
|
Multiple myeloma, etc.
|
Amgen
|
|
PSMAxCD3
|
PSMA/CD3
|
I
|
Prostate cancer, etc.
|
Pfizer
|
|
CEAxCD3
|
CEA/CD3
|
I
|
Colon cancer, etc.
|
Pfizer
|
scDiabody drugs have shown some advantages and challenges in clinical settings. The advantages of scDiabody drugs include high efficiency in redirecting T cells to tumor cells, inducing strong cytotoxic responses, and inhibiting tumor growth and metastasis. They also exhibit low toxicity and side effects. scDiabody drugs can reduce the damage to normal tissues and blood clearance due to their high specificity and low molecular weight, reducing damage to normal tissues. Additionally, scDiabody drugs are easily producible through gene engineering and prokaryotic expression systems, thanks to their simple structure and fewer connection sites.
References
1. Holliger P, et al. Engineered antibody fragments and the rise of single domains. Nat Biotechnol. 2005;23(9):1126-1136.
2. Wu Z, et al. T cell engaging bispecific antibody (T-BsAb): from technology to therapeutics. Pharmacol Ther. 2018;182:161-175.
3. Kontermann RE, et al. Bispecific antibodies. Drug Discov Today. 2015;20(7):838-847.
4. Spiess C, et al. Alternative molecular formats and therapeutic applications for bispecific antibodies. Mol Immunol. 2015;67(2 Pt A):95-106.
5. Chames P, et al. Therapeutic antibodies: successes, limitations and hopes for the future. Br J Pharmacol. 2009;157(2):220-233.
6. Li J, et al. Advances in developing novel therapeutic strategies for cancer immunotherapy: bispecific antibodies and chimeric antigen receptor T cells. Cancer Commun (Lond). 2019;39(1):22.
7. Zhu M, et al. Bispecific antibodies for cancer immunotherapy: current perspectives. Biosci Rep. 2019;39(12):BSR20192860.
8. Fan G, et al. Bispecific antibodies and their applications. J Hematol Oncol. 2015;8:130.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY