What is scFv-CH-CL-scFv
scFv-CH-CL-scFv is a recombinant antibody composed of two single-chain antibodies (scFv) connected by a flexible linker peptide, each scFv contains a variable light chain (VL) and a variable heavy chain (VH) that can bind to antigen. scFv-CH-CL-scFv is a product of a novel antibody engineering technology that overcomes some limitations of traditional monoclonal antibodies (mAb) and single-chain antibodies (scFv), such as smaller molecular weight, better tissue penetration ability, faster blood clearance rate, lower immunogenicity, etc. scFv-CH-CL-scFv is mainly used for cancer immunotherapy, by constructing chimeric antigen receptor (CAR) to redirect T cells, enabling them to recognize and kill tumor cells.
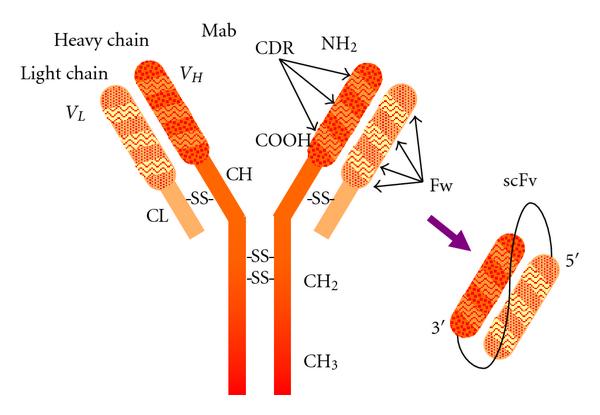
Fig.1 Antibody model showing subunit composition and domain distribution along the polypeptide chains. (Ahmad ZA, 2012)
scFv-CH-CL-scFv has a broad application prospect in cancer immunotherapy, especially in the construction of chimeric antigen receptor (CAR). CAR is an artificially synthesized protein consisting of an exogenous antigen recognition structure (usually an scFv or scFv-CH-CL-scFv), a transmembrane structure and an endogenous signal transduction structure. CAR can be transduced to T cells, enabling them to directly recognize and kill tumor cells expressing specific antigens without the mediation of major histocompatibility complex (MHC). Currently, several CAR-T cell therapy products based on CD19-specific scFv or scFv-CH-CL-scFv have been approved by the US Food and Drug Administration (FDA) for the treatment of relapsed or refractory B-cell malignancies. In addition, there are many CAR-T cell therapy products based on other targets of scFv or scFv-CH-CL-scFv in different stages of clinical trials, showing good safety and efficacy.
Structural Features of scFv-CH-CL-scFv
The structure of scFv-CH-CL-scFv consists of two scFvs linked by a flexible peptide linker, each scFv contains a VL and a VH domain composed of a V and a J region. The linker length, sequence and solubility affect the stability, folding and aggregation tendency of scFv-CH-CL-scFv. The linker also keeps the VL and VH domains at an appropriate distance to facilitate proper folding and antigen-binding site formation, while preventing excessive oligomerization of scFv-CH-CL-scFv. The relative position of VL and VH domains is another factor that influences the structure of scFv-CH-CL-scFv. There are two possible configurations: VL-linker-VH or VH-linker-VL. Both configurations can generate functional scFv-CH-CL-scFv, but different scFvs may perform better in different configurations. Therefore, the effect of VL and VH domain orientation on the structure and function of scFv-CH-CL-scFv should be considered in the design process.
Generation Methods of scFv-CH-CL-scFv
The generation methods of scFv-CH-CL-scFv mainly include genetic engineering and in vitro display. Genetic engineering involves connecting two scFv genes with a linker sequence and expressing them in bacteria, using known or available scFv genes against a specific antigen. In vitro display involves screening and linking scFvs with high affinity and specificity from a large scFv library containing diverse variable region combinations, using display vectors such as phage or yeast.
Clinical Data of scFv-CH-CL-scFv
The clinical application of scFv-CH-CL-scFv is mainly reflected in the construction of chimeric antigen receptor (CAR), and currently there are several CAR-T cell therapy products based on scFv-CH-CL-scFv that have been approved by the US Food and Drug Administration (FDA) for the treatment of relapsed or refractory B-cell malignancies. In addition, there are many CAR-T cell therapy products based on scFv-CH-CL-scFv in different stages of clinical trials, targeting different tumor antigens, showing good safety and efficacy.
Table.1 examples of approved products based on scFv-CH-CL-scFv
|
Product name
|
Target
|
Approval date
|
Indication
|
Population
|
Country/region
|
|
Kymriah (tisagenlecleucel)
|
CD19
|
Aug-17
|
Acute lymphoblastic leukemia (ALL)
|
Children and adolescents
|
USA, EU, Japan, Canada, etc.
|
|
Yescarta (axicabtagene ciloleucel)
|
CD19
|
Oct-17
|
Relapsed or refractory diffuse large B-cell lymphoma (DLBCL)
|
Adults
|
USA, EU, Japan, Canada, etc.
|
|
Tecartus (brexucabtagene autoleucel)
|
CD19
|
Jul-20
|
Relapsed or refractory mantle cell lymphoma (MCL)
|
Adults
|
USA
|
|
Breyanzi (lisocabtagene maraleucel)
|
CD19
|
Feb-21
|
Relapsed or refractory diffuse large B-cell lymphoma (DLBCL)
|
Adults
|
USA
|
|
Abecma (idecabtagene vicleucel)
|
BCMA
|
Mar-21
|
Relapsed or refractory multiple myeloma (MM)
|
Adults
|
USA
|
Table.2 examples of clinical trial products based on scFv-CH-CL-scFv
|
Product name
|
Target
|
Clinical trial phase
|
Main sponsor
|
|
MOR208 (tafasitamab)
|
CD19
|
Phase III (NCT04140536)
|
MorphoSys AG
|
|
MOR202 (lenalidomide)
|
CD38
|
Phase II (NCT01421186)
|
MorphoSys AG
|
|
MOR106 (IL17C)
|
IL17C
|
Phase II (NCT03815580)
|
MorphoSys AG
|
|
MOR107 (Lanthipeptide)
|
Lanthipeptide receptor 2 (LANCL2)
|
Phase I (NCT03289661)
|
MorphoSys AG
|
References
1. Ahmed M, et al. Advantages of scFv antibodies in cancer therapy. Antibodies (Basel). 2012 Dec 21;2(1):70-88.
2. Bates N, Power CA. Single-chain variable fragments: production and applications in cancer therapy. Methods Mol Biol. 2019;1904:1-24.
3. Bradbury ARM, et al. When are scFvs sufficient for antibody-mediated therapies? Methods Mol Biol. 2011;748:245-58.
4. Ahmad ZA, et al. scFv antibody: principles and clinical application. Clin Dev Immunol. 2012;2012:980250.
5. Iizuka A, et al. A T-cell-engaging B7-H4/CD3-bispecific Fab-scFv Antibody Targets Human Breast Cancer. Clin Cancer Res. 2019 May 1;25(9):2925-2934.
6. Monnier PP, et al. Single-chain antibody fragments for ocular therapeutic applications: prospects and challenges. Adv Drug Deliv Rev. 2013 Nov;65(11):1496-513.
7. Sandomenico A, et al. Optimization of scFv antibody fragments for tumor targeting. J Biotechnol. 2020 Jan 20;307:108-121.
8. FDA approves lisocabtagene maraleucel for relapsed or refractory large B-cell lymphoma [Internet]. U.S. Food and Drug Administration. FDA; [cited 2021Dec16]. Available from: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-lisocabtagene-maraleucel-relapsed-or-refractory-large-b-cell-lymphoma
9. FDA approves idecabtagene vicleucel for multiple myeloma [Internet]. U.S. Food and Drug Administration. FDA; [cited 2021Dec16]. Available from: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-idecabtagene-vicleucel-multiple-myeloma
10. Bird RE, et al. Single-chain antigen-binding proteins. Science. 1988 Oct 7;242(4877):423-6.
11. Huston JS, et al. Protein engineering of antibody binding sites: recovery of specific activity in an anti-digoxin single-chain Fv analogue produced in Escherichia coli. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5879-83.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY