Intrabodies are antibodies or antibody fragments that can function within the cell to bind to an intracellular protein. They are a method that can directly interfere with intracellular target proteins, helping to reveal disease mechanisms and develop new therapeutic strategies. The research on intrabodies originated in the 1990s, when scientists discovered a special type of heavy-chain antibody produced by camelids, whose variable region can exist as a stable antibody fragment with high affinity, specificity and permeability. Later, single-chain variable fragments (scFv) from human or mouse were also used to construct intrabodies, by linking the variable regions of heavy and light chains (VH and VL) to form a single-chain antibody fragment.
Structural Features of Intrabodies
The general structure of intrabodies consists of variable regions (VH or VL), linker peptides (linker) and tags (tag). The variable regions are the antigen-binding sites of antibodies, which determine the affinity and specificity of intrabodies. The linker peptides are short peptide segments that connect VH and VL, usually 15 amino acids of (GGGGS)3 sequence, which ensure the flexibility and stability of intrabodies. The tags are short peptide sequences attached to the end of the variable regions, which can guide the intracellular localization and transport of intrabodies, such as nuclear localization signals (NLS), endoplasmic reticulum retention signals (KDEL), etc.
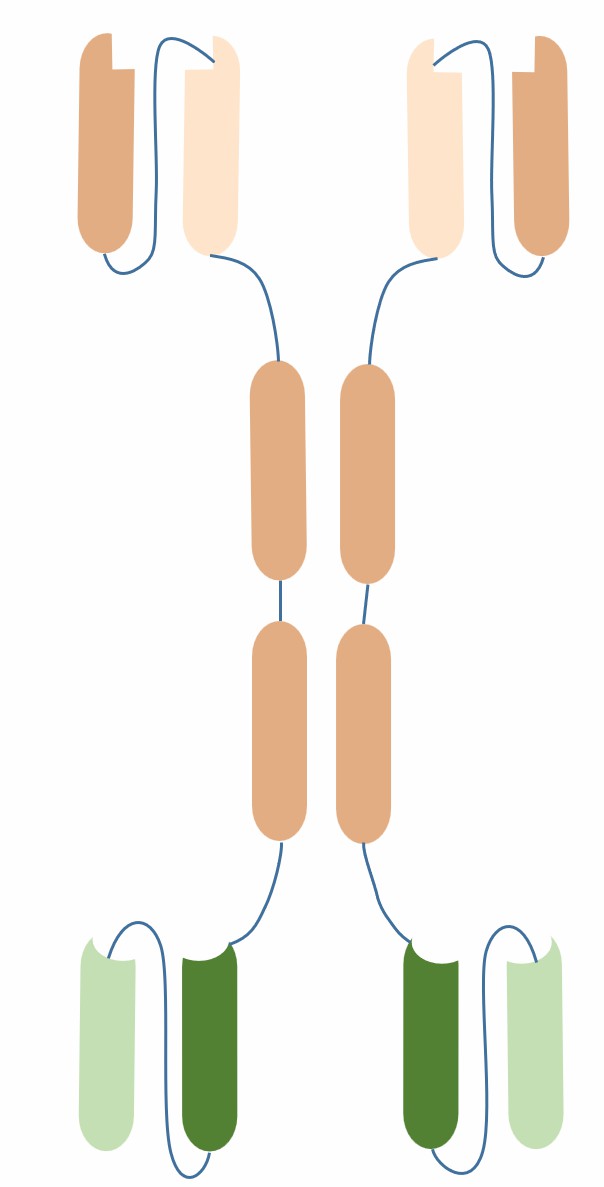
Fig.1 Schematic diagram of intrabody (Creative Biolabs)
How the structure of intrabodies affects their intracellular expression, localization, transport and function is an important research topic. On the one hand, intrabodies need to adapt to the complex and variable intracellular environment, such as redox state, temperature, pH value, etc. On the other hand, intrabodies need to act precisely on specific target proteins, avoiding interaction or aggregation with other non-specific proteins. Therefore, rational design and optimization of the structure of intrabodies is the key to improve their efficiency and safety.
Generation Methods of Intrabody
The common methods for generating intrabodies include phage display, yeast display, aptamers, etc. These methods are based on high-throughput screening techniques, which can select intrabodies with high affinity and specificity from large antibody libraries. Phage display is the earliest and most widely used method, which uses phage surface proteins to display antibody fragments, and infects host bacteria to amplify and express them. Yeast display is another common method, which uses yeast surface proteins to display antibody fragments, and relies on yeast cell growth and division to amplify and express them. Aptamers are a novel method, which uses artificially synthesized nucleic acid molecules to mimic antibody fragments, and relies on nucleic acid replication and mutation to amplify and optimize them.
To optimize the affinity and specificity of intrabodies, the generation methods can be improved or combined. For example, intrabodies can be engineered by mutation, humanization, fusion, etc., to improve their binding ability to target proteins and avoid immune responses. Alternatively, multiple display systems or antibody libraries can be used to increase the diversity and coverage of intrabodies, and improve their screening success rate.
Intrabody-Related Clinical Data
Intrabodies have broad application prospects and progress in the fields of neurodegenerative diseases, viral infections and tumor immunotherapy. Intrabodies can target some intracellular pathogenic proteins, such as α-synuclein in Parkinson's disease, amyloid-β in Alzheimer's disease or mutant huntingtin in Huntington's disease, and improve neuronal function and survival by inhibiting their expression, aggregation or toxicity. Intrabodies can also target some proteins associated with viral infections, such as HIV TAT, Rev, Vif, gp41, gp120, gp160, etc., and inhibit viral replication or escape by blocking their interaction with host cells or other viral proteins. Intrabodies can also target some proteins related to tumor occurrence and development, such as p53, HER2, STAT3, RHOB, cortactin, VEGFR2, Ras, Bcr-Abl, etc., and inhibit tumor growth or spread by regulating their signal transduction, cell cycle, apoptosis, metastasis, angiogenesis or immune recognition.
Some intrabody products have been approved or are in clinical trials. These products are targeted at extracellular or secreted target proteins. They treat some blood system- or immune system-related diseases by retaining them in the endoplasmic reticulum (ER) or secreting them into the blood.
Table 1. Approved intrabody products
|
Product name
|
Main target
|
Approval time
|
Indication
|
Population
|
Country/region
|
|
Efgartigimod (ARGX-113)
|
FcRn receptor
|
2021 (US), 2022 (EU)
|
Myasthenia gravis (MG), immune thrombocytopenic purpura (ITP), dermatomyositis (DM), chronic inflammatory demyelinating polyneuropathy (CIDP)
|
Adults with generalized MG, adults with primary ITP, adults with DM, adults with CIDP
|
US, EU
|
The ongoing or planned clinical trials related to intrabodies are mainly focused on products targeting intracellular target proteins, such as Ras protein family (KRAS, NRAS, HRAS), etc. These products use gene therapy methods to introduce intrabodies into target cells, and treat some solid tumors that are difficult to treat with conventional drugs by interfering with the function of intracellular target proteins, such as pancreatic cancer and colorectal cancer.
Table 2. Ongoing or planned intrabody trials
|
Trial name
|
Main target
|
Trial phase
|
Trial purpose
|
Trial design
|
|
A Phase I/II Study of Intratumoral Injection of ERAS-007 in Patients With Locally Advanced Pancreatic Cancer After FOLFIRINOX and Chemoradiation Therapy (ERAS-007-PANC01)
|
KRAS protein family (KRAS, NRAS, HRAS)
|
Phase I/II
|
To evaluate the safety, tolerability, pharmacokinetics, pharmacodynamics and preliminary efficacy of ERAS-007 in patients with locally advanced pancreatic cancer after FOLFIRINOX and chemoradiation therapy.
|
Open-label, single-arm, dose-escalation and dose-expansion study.
|
|
A Phase I/II Study of Intratumoral Injection of ERAS-007 in Patients With KRAS Mutant Colorectal Cancer After Progression on First-line Therapy With an Anti-EGFR Antibody Plus Chemotherapy (ERAS-007-CRC01)
|
KRAS protein family (KRAS, NRAS, HRAS)
|
Phase I/II
|
To evaluate the safety, tolerability, pharmacokinetics, pharmacodynamics and preliminary efficacy of ERAS-007 in patients with KRAS mutant colorectal cancer after progression on first-line therapy with an anti-EGFR antibody plus chemotherapy.
|
Open-label, single-arm, dose-escalation and dose-expansion study.
|
References
1. Lo AS-Y, et al. Intracellular antibodies (intrabodies) and their therapeutic potential. Handb Exp Pharmacol. 2008;(181):343-73.
2. Lo AS-Y, et al. Intracellular Antibodies (Intrabodies) and Their Therapeutic Potential. In: Kontermann R, Dübel S, editors. Therapeutic Antibodies. Berlin, Heidelberg: Springer; 2009. p. 343–73.
3. Zhang C, et al. Applying Antibodies Inside Cells: Principles and Recent Advances in Neurobiology, Virology and Oncology. BioDrugs. 2020 Jun;34(3):435–62.
4. Cohen PA. Targeting scFv Expression to Eukaryotic Intracellular Compartments. In: Kontermann R, Dübel S, editors. Antibody Engineering: Methods and Protocols. Totowa, NJ: Humana Press; 2004. p. 367–81.
5. Marschall ALJ, et al. Designing Intrabodies: Structural Features and the Use of Intracellular Trafficking Signals. In: Lo AS-Y, Marasco WA, editors. Intrabodies: Basic Research and Clinical Gene Therapy Applications. Berlin, Heidelberg: Springer; 2000. p. 1–22.
6. Rondon IJ, et al. Intracellular antibodies (intrabodies) for gene therapy of infectious diseases. Annu Rev Microbiol. 1997;51:257-83.
7. Yu J, et al. Optogenetic control of intracellular antibodies (intrabodies) for tunable protein blockage in living cells. Nat Methods. 2020 Mar;17(3):271-278.
8. Wang Q, et al. Design and Production of Bispecific Antibodies. Antibodies (Basel). 2019 Aug 2;8(3):43.
9. Aires da Silva F, et al. Intrabody-based gene therapy strategies for the treatment of HIV/AIDS. Expert Opin Biol Ther. 2011 Nov;11(11):1485-97.
10. Coloma MJ, et al. Novel vectors for expression of antibody molecules using variable regions generated by polymerase chain reaction. J Immunol Methods. 1992 May 4;152(1):89-104.
11. Biocca S, et al. Expression and targeting of intracellular antibodies in mammalian cells. EMBO J. 1990 Dec;9(1):101-8.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY