What is SdAb-HAS
SdAb-HAS is a fusion protein composed of single-domain antibody (sdAb) and human serum albumin (HAS), which has potent antitumor activity. SdAb is the variable domain of heavy chain antibody derived from camelids, which is the smallest natural antibody fragment known so far, with a size of about 15 kDa. SdAb has many advantages over conventional monoclonal antibodies (mAb), such as high affinity, high stability, high solubility, etc. However, sdAb also has some drawbacks, such as short half-life, low pharmacokinetic parameters, low potency, etc. To overcome these drawbacks, different strategies have been adopted to improve the drug properties of sdAb, one of which is to link sdAb with HAS, forming sdAb-HAS. HAS is a plasma protein widely distributed in the human body, which has the functions of prolonging circulation time, increasing drug load, reducing renal excretion, reducing toxic side effects, etc. By linking sdAb with HAS, the therapeutic effect and safety of sdAb can be improved.
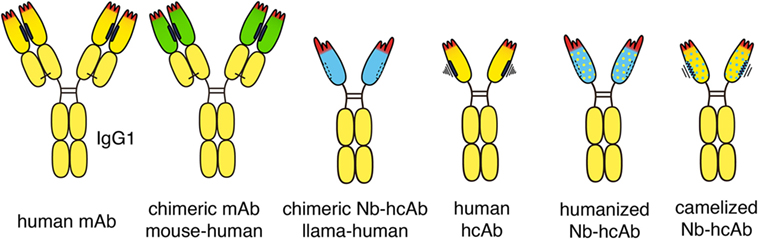
Figure.1 Chimeric and humanized heavy chain anti-tumor antibodies
Generation Methods of SdAb-HAS
The common generation methods of sdAb and sdAb-HAS include the following steps: immunization, library construction, selection, expression and purification. These steps can be optimized and adjusted according to different target antigens and application requirements. Each method has its advantages and limitations, and needs to be selected and combined according to the actual situation. Immunization refers to the use of target antigens to immunize camelids, such as camels, alpacas or llamas, to induce them to produce specific heavy chain antibodies. The advantage of immunization is that it can obtain sdAbs against self or difficult-to-prepare antigens, as well as sdAbs against different epitopes or conformations. Library construction requires the use of specific primers to amplify the heavy chain antibody variable region genes, and insert them into vectors with display proteins, such as the pIII protein of phage or the AGA2 protein of yeast. The purpose of library construction is to obtain a library with high diversity and coverage for subsequent selection. Library construction can obtain high-quality and large libraries, and also increase the diversity and complexity of libraries by synthesis or mutation. However, it requires optimizing the quality and size of the library, avoiding false positives and negatives. Selection means to the use of target antigens or cells for multiple rounds of selection, screening out candidates with high affinity and specificity. After selection, sdAbs with high affinity and specificity can be obtained. Also sdAbs against different epitopes or conformations can be obtained by competition or co-selection. Nevertheless, it requires optimizing the selection conditions and strategies, avoiding cross-reaction and non-specific binding. Expression and purification refer to cloning the candidate sdAb genes into expression vectors and expressing them in host cells. After expression, the target sdAb needs to be purified by affinity chromatography or other methods. Binding HAS can use different ways and sites, such as N-terminal, C-terminal, internal insertion. The purpose of binding HAS is to extend the half-life and distribution of sdAb in vivo. The advantage of binding HAS is that it can improve the pharmacokinetic characteristics of sdAb, and also increase the multivalency or multitargeting of sdAb-HAS by adjusting the binding ratio or order. The disadvantage of binding HAS is that it requires optimizing the binding way and site, avoiding structural changes and functional effects.
Clinical Data of SdAb-HAS
Currently, only one sdAb-HAS product has been approved by EMA or FDA, which is for the treatment of adult acquired thrombotic thrombocytopenic purpura (TTP). TTP is a rare blood disorder characterized by low platelet count, microvascular thrombosis and organ damage.
Currently, there are several sdAb-HAS in different stages of clinical trials, mainly targeting cancer, neurodegenerative diseases, infectious diseases and others. These sdAb-HAS are mainly developed by different companies or research institutions, such as Ablynx, Sanofi, Genzyme, Novartis and others.
Table 1. Summary of sdAb-HAS in clinical trials
|
Target
|
Indication
|
Clinical trial phase
|
Developer
|
|
RANKL
|
Osteoporosis
|
III
|
Ablynx/Sanofi
|
|
IL-6R
|
Rheumatoid arthritis
|
III
|
Ablynx/Sanofi
|
|
TNF-α
|
Rheumatoid arthritis, Crohn's disease, ulcerative colitis and others
|
II/III
|
Ablynx
|
|
EGFR
|
Solid tumors
|
II/III
|
Ablynx
|
|
CD47/SIRPα
|
Hematological malignancies and solid tumors
|
I/II
|
Ablynx
|
|
MMP12
|
Chronic obstructive pulmonary disease (COPD)
|
I/II
|
Ablynx
|
|
MMP14
|
Solid tumors and fibrotic diseases
|
I/II
|
Ablynx
|
|
MMP9/NGAL
|
Rasmussen encephalitis and other neurodegenerative diseases
|
I/II
|
Ablynx
|
|
MICA/B/NKG2D
|
Solid tumors and hematological malignancies
|
I/II
|
Ablynx
|
References
1. Jin BK, et al. Single-domain antibodies (sdAbs): A Review of Generation, Diagnostics and Therapeutics. Int J Mol Sci. 2023 Mar 22;24(6):5994.
2. Bannas P, et al. Single-domain antibodies (sdAbs) and single-domain antibody (sdAb)-based human heavy chain antibodies as antitumor therapeutics. Front Immunol. 2017 Nov 22;8:1603.
3. Jovčevska I, et al. The therapeutic potential of single-domain antibodies (sdAbs). BioDrugs. 2020 Feb;34(1):11-26.
4. Wang J, et al. Research progress and applications of multivalent, multispecific and modified single-domain antibodies (sdAbs) for disease treatment. Front Immunol. 2021 Jul 22;12:838082.
5. De Meyer T, et al. single-domain antibody (sdAb)
-based products as research and diagnostic tools. Trends Biotechnol. 2014 May;32(5):263-70.
6. Roovers RC, et al. Efficient inhibition of EGFR signaling and of tumour growth by antagonistic anti-EFGR Single-domain antibodies (sdAbs). Cancer Immunol Immunother. 2007 Mar;56(3):303-17.
7. Cunningham A, et al. single-domain antibody (sdAb)-based anti-RSV prophylaxis and therapy: preclinical proof of concept for a bispecific anti-F/G single-domain antibody (sdAb) with improved in vivo half-life. Antiviral Res. 2021 Feb;186:104999.
8. Hanke L, et al. Single-domain antibodies (sdAbs) from camelid mice and llamas neutralize SARS-CoV-2 variants. Nature. 2021 Aug;596(7872):415-420.
9. De Genst EJ, et al. Single-domain antibodies (sdAbs) targeting the amyloid β peptide can inhibit amyloid formation without increasing neurotoxicity - a strategy for the treatment of Alzheimer's disease? Biochem Soc Trans. 2019 Dec 20;47(6):1739-1748.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY