Allograft Rejection Detection-Applied Exosomes in Organ Transplantation
Overview Services Features FAQs
Exosomes and the donor-specific molecules they carry can be used as diagnostic markers for the detection of allograft immune rejection that affects the survival rate of recipients after organ transplantation, overcoming the problem of poor sensitivity of traditional detection methods. As a leading researcher in the field of exosomes, Creative Biolabs is committed to providing clients with research services related to the application of exosomes for the diagnosis of allograft rejection, to advance the research progress of personalized and precise diagnosis of organ transplantation.
Advances in the Detection of Allograft Rejection
-
To precisely suggest the exact etiology of rejection, traditional assays are of use, including memory B cells, DSA (donor-specific antibodies), regulatory immune cells, and follicular helper T cells that mediate B cell proliferation and differentiation. Detection of immune status after organ transplantation by using CD4+ T cells, CD8+ T cells, and CD4/CD8 T cell ratio can be used to assess the occurrence of rejection.
-
However, the poor specificity and sensitivity of the indicators lead to abnormal indicator responses lagging behind the irreversible damage that has already occurred in the organ, such as a negative serum DSA indicator in a patient whose pathological biopsy shows the occurrence of rejection of the transplanted organ. This impedes the control and performance of the desired therapeutic effect of organ transplantation.
-
Allograft rejection involves exosomes of donor graft origin carrying donor antigens from antigen-presenting cells to recipient lymphoid tissue and functioning as mediators of T-cell activation. Therefore, exosomes can be applied for early diagnosis and longitudinal detection of immune rejection and are important biomarkers for responding to the physiological status of the recipient after organ transplantation, with the benefits of accuracy, convenience, and non-invasive.
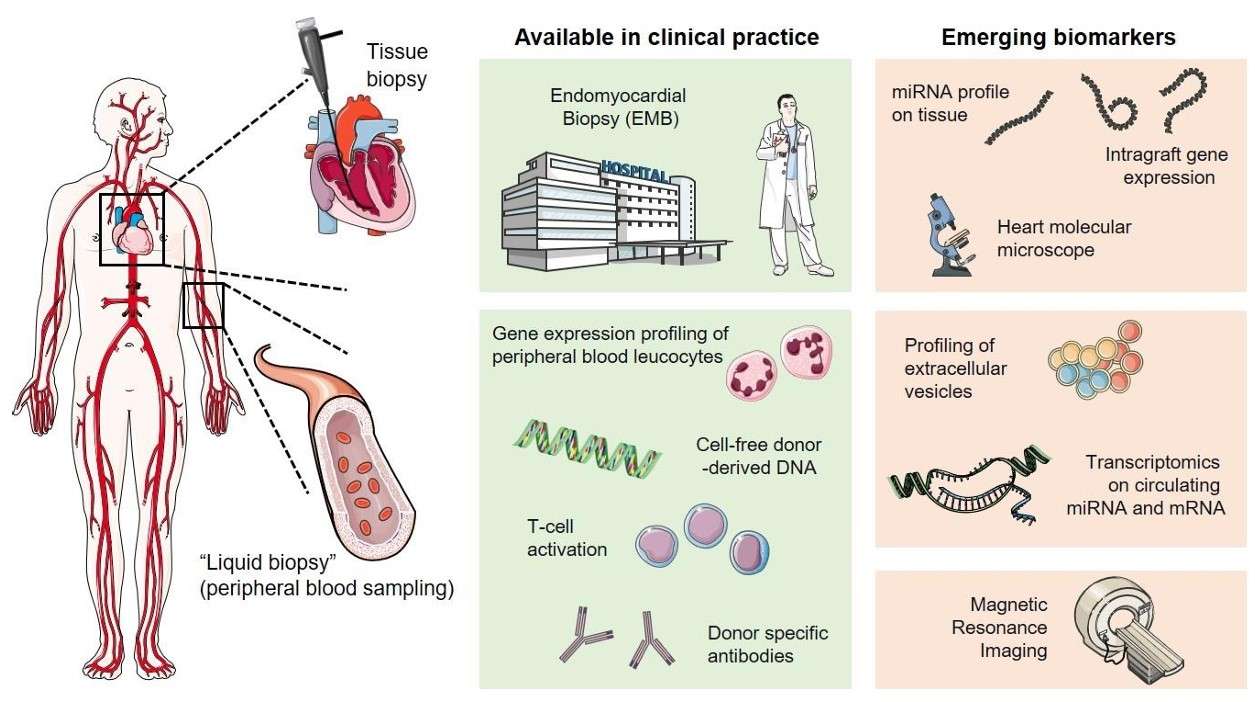 Fig. 1 Overview of invasive and non-invasive approaches in cardiac allograft rejection monitoring.1
Fig. 1 Overview of invasive and non-invasive approaches in cardiac allograft rejection monitoring.1
Allograft Rejection Detection-Applied Exosomes in Creative Biolabs
-
Donor-derived specific exosomes maintain some of the biological properties of the donor and may serve as a novel biomarker resource for allograft rejection. For example, the detection of immature dendritic cell-derived exosomes may respond to their induction of immune tolerance in cardiac grafts, while regulatory T cell (Treg)-derived exosomes are used to assess the survival time of transplanted kidneys and the damage to kidney tissue by reduced immune cells.
-
The cargo carried by exosomes causes changes associated with the onset of immune rejection and is another important basis for detecting allograft rejection. For example, studies have detected exosomes expressing autoantigens such as myosin and wave proteins in sera from the rejection group of heart transplant animal experiments. Exosomes of peripheral blood origin from patients with occlusive fine bronchiectasis syndrome express donor human leukocyte antigens, lung autoantigens, and cell adhesion molecules on their surface after lung transplantation.
-
An elevated proportion of IL-21-producing Tfh-derived exosomes can be detected in peripheral blood after renal transplant rejection, accompanied by significant changes in cytotoxic T lymphocyte-associated antigen-4 on the surface of exosomes. Serum cfDNA is derived from exosomes, and 93% of plasma DNA accumulates in exosomes. Analysis of cfDNA levels in exosomes can be used to assess the stability of graft function.
-
Although a small percentage of Treg, follicular regulatory T cells, and regulatory plasma cells are present in peripheral blood changes that occur after the onset of rejection are difficult to detect significantly. It is promising that their derived exosomes and specific markers can be used as a diagnostic basis with high sensitivity and in contrast, have a better application in response to allograft rejection.
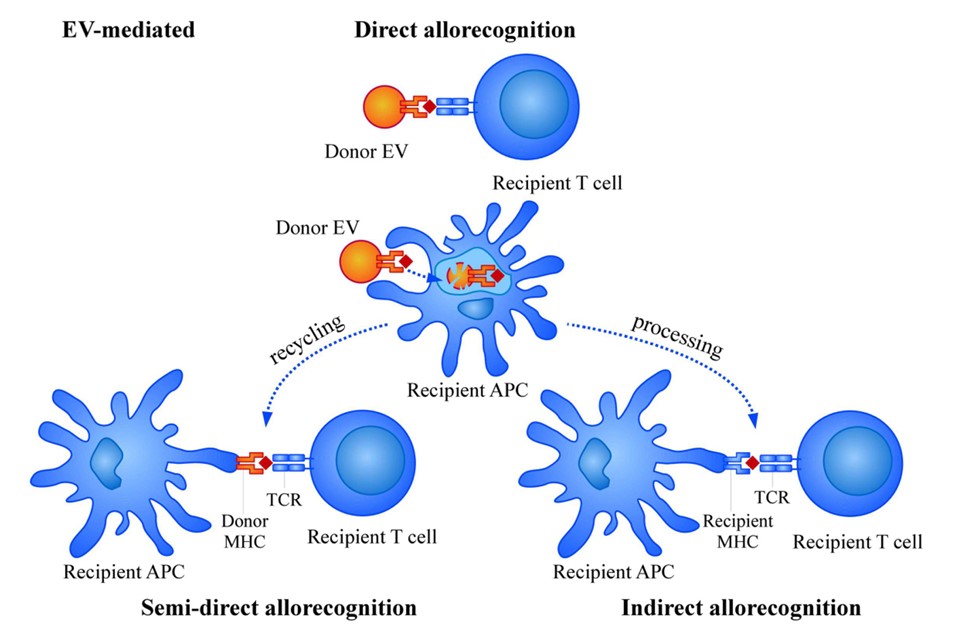 Fig. 2 Mechanisms of allorecognition by EVs.2
Fig. 2 Mechanisms of allorecognition by EVs.2
Features
-
Early Detection: Exosomes can provide early warning signals of allograft rejection, allowing for prompt intervention and improved patient outcomes.
-
Non-Invasive: The use of exosomes for detecting rejection is non-invasive, involving simple blood or urine tests instead of more invasive biopsy procedures.
-
High Sensitivity and Specificity: Exosome-based assays can detect subtle changes in graft status with high sensitivity and specificity, making them a reliable monitoring method.
-
Biomarker Potential: Exosomes carry a variety of biomarkers such as proteins, RNAs, and lipids that are indicative of immune responses and graft health.
-
Real-Time Monitoring: Continuous or regular monitoring using exosome analysis allows for real-time assessment of graft condition, leading to better management of organ transplantation.
To discover more exosome-related biomarkers that meet the need to advance diagnostic and risk prediction studies of rejection, Creative Biolabs offers comprehensive exosome isolation and analysis solutions to help clients investigate the use of exosomes in the diagnosis of allogeneic transplant rejection. Please feel free to contact us.
FAQs
Q: How do exosomes help in detecting allograft rejection?
A: Exosomes shed by immune cells or graft cells can carry specific biomarkers that indicate the presence of rejection processes. Analyzing these biomarkers in body fluids allows for early and accurate detection of graft rejection.
Q: Is exosome-based rejection detection more accurate than traditional methods?
A: Exosome-based detection methods are highly sensitive and specific, and can often detect rejection earlier than traditional biopsy-based methods. For thorough monitoring, it's crucial to combine these techniques with additional diagnostic instruments.
Q: Which kinds of samples are required for the examination of exosomes?
A: Exosome analysis typically requires non-invasive samples such as blood or urine. These samples are processed to isolate exosomes, which are then analyzed for rejection biomarkers.
Q: Can exosome-based detection be used for all types of organ transplants?
A: Indeed, while study and validation are still being done for different organ types, exosome-based detection has the potential to be used for a variety of organ transplants, including kidney, liver, heart, and lung transplants.
References
-
Giarraputo, Alessia, et al. "A changing paradigm in heart transplantation: an integrative approach for invasive and non-invasive allograft rejection monitoring." Biomolecules 11.2 (2021): 201. Under Open Access license CC BY 4.0, without modification.
-
Monguió-Tortajada, Marta, Ricardo Lauzurica-Valdemoros, and Francesc E. Borràs. "Tolerance in organ transplantation: from conventional immunosuppression to extracellular vesicles." Frontiers in immunology 5 (2014): 416. Under Open Access license CC BY 4.0. The image was modified by revising the title.
For Research Use Only. Cannot be used by patients.
Related Services:

 Fig. 1 Overview of invasive and non-invasive approaches in cardiac allograft rejection monitoring.1
Fig. 1 Overview of invasive and non-invasive approaches in cardiac allograft rejection monitoring.1
 Fig. 2 Mechanisms of allorecognition by EVs.2
Fig. 2 Mechanisms of allorecognition by EVs.2









