Asparagus cochinchinensis-derived Exosome Research & Application
Overview Advantages Research Potentiality Services Products FAQs
Nature's Nanomedicine: A New Paradigm from Asparagus cochinchinensis
The field of nanomedicine is undergoing a paradigm shift, moving from synthetically engineered nanoparticles towards nature-derived exosomes for therapeutic delivery. While liposomes and polymeric nanoparticles have shown promise, challenges related to immunogenicity, biocompatibility, and complex manufacturing have limited their clinical translation. In this context, exosomes derived from plants (PDExos) have emerged as a highly compelling alternative. These vesicles, secreted by plant cells, are integral to intercellular communication and defense mechanisms, carrying a cargo of bioactive lipids, proteins, and nucleic acids.
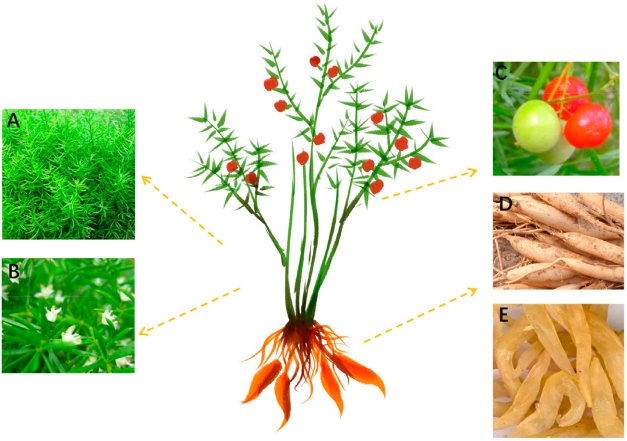 Fig.1 A. cochinchinensis plant morphology.1
Fig.1 A. cochinchinensis plant morphology.1
Among the vast flora with medicinal properties, Asparagus cochinchinensis, a perennial herb deeply rooted in traditional medicine, presents a unique and untapped resource. Historically valued for its anti-inflammatory, antioxidant, and anti-tumor properties, this plant offers the potential for its exosomes to be not merely passive drug carriers, but potent therapeutic agents in their own right. The investigation into Asparagus cochinchinensis-derived exosomes (ACExos) represents a convergence of ancient herbal wisdom and cutting-edge nanotechnology, opening a new frontier for developing safer, more effective treatments for complex diseases such as cancer.
Explore the Potential of ACExos — Connect with Our Experts Today.
The Inherent Edge: Biocompatibility Meets Bioactivity
The therapeutic potential of ACExos is underpinned by a suite of intrinsic advantages derived from their botanical origin and unique biochemical composition. These benefits position them as a superior platform for next-generation nanotherapeutics.
Natural Safety
Derived from the edible medicinal plant Asparagus cochinchinensis, ACExos show excellent biocompatibility and low immunogenicity, suitable for repeated use.
Bioactive Cargo
Naturally enriched with saponins, polysaccharides, and amino acids, ACExos possess intrinsic antioxidant and anti-inflammatory functions.
Efficient Delivery
Their nanoscale structure ensures stable cargo protection, efficient cellular uptake, and flexible surface modification for targeted delivery.
Sustainable Production
Plant-based sourcing enables scalable, cost-effective, and eco-friendly manufacturing, supporting both research and commercial applications.
Partner with Us to Advance Your Exosome Research.
Scientific Validation: From Laboratory to Preclinical Success
Foundational research has begun to validate the therapeutic potential of Asparagus cochinchinensis–derived exosomes (ACExos), particularly in oncology. A pivotal study, laid the groundwork for this innovative therapeutic platform. In this work, researchers successfully isolated and purified ACExos through a rigorous process combining differential centrifugation and sucrose density gradient ultracentrifugation. Characterization confirmed their exosome nature, showing a cup-shaped morphology (~119 nm) and a biochemical composition rich in lipids, proteins, and RNA.
|
Feature
|
Description
|
|
Morphology
|
Cup-shaped vesicles observed under TEM
|
|
Average Diameter
|
~119 nm
|
|
Composition
|
Lipids, proteins, and RNA
|
|
Isolation Method
|
Differential centrifugation + sucrose gradient ultracentrifugation
|
Functionally, ACExos exhibited a potent anti-proliferative effect against hepatocellular carcinoma (HepG2) cells. This activity was mediated by apoptosis induction, a critical pathway for eliminating malignant cells. Mechanistic studies further revealed that ACExos are internalized mainly through phagocytosis, ensuring effective cellular uptake by tumor cells.
However, in vivo experiments uncovered a pharmacokinetic limitation—unmodified ACExos were rapidly cleared from circulation by scavenger receptors in the liver and spleen. To address this, the research team engineered PEGylated ACExos, coating the vesicle surface with polyethylene glycol (PEG) to evade immune recognition and prolong systemic circulation. This modification markedly enhanced tumor accumulation via the enhanced permeability and retention (EPR) effect.
|
Modification
|
Objective
|
Outcome
|
|
PEGylation
|
Reduce rapid clearance
|
Extended blood circulation time
|
|
Enhance tumor targeting
|
Improved passive accumulation (EPR effect)
|
Therapeutic assessment in a HepG2 xenograft mouse model demonstrated that PEG-ACExos significantly inhibited tumor growth while maintaining an excellent safety profile—stable body weight and normal histology of major organs.
This study provides the first robust evidence that ACExos can be engineered into a safe and effective nanotherapeutic platform for cancer treatment, representing a promising convergence of plant biology and nanomedicine.
Unlock the Science Behind ACExos. Schedule a Consultation.
Charting the Therapeutic Horizon: Beyond Oncology
The established anti-HCC activity of ACExos is likely just the vanguard of their broader therapeutic potential. The unique combination of a biocompatible delivery vehicle and an intrinsic bioactive payload opens avenues for application across a spectrum of diseases.
 Oncology
Oncology
Demonstrated anti-HCC effects suggest broader potential in colorectal, pancreatic, and lung cancers, either as standalone therapeutics or synergistic drug carriers.
 Targeted Delivery
Targeted Delivery
Natural liver tropism and modifiable surface ligands enable precise targeting to specific cells or tissues.
 Inflammatory Disorders
Inflammatory Disorders
Intrinsic anti-inflammatory and immunomodulatory activity supports applications in IBD, arthritis, and sepsis.
 Dermatology
Dermatology
Rich antioxidants and bioactives promote skin protection, repair, and rejuvenation, offering promise in anti-aging and skin disorder treatments.
Transform Your Ideas into Results — Request a Project Evaluation.
Accelerating Your Research: Our Services
At Creative Biolabs, we have developed a comprehensive, end-to-end platform to accelerate the research and development of Asparagus cochinchinensis-derived exosomes. Our suite of services empowers our clients to seamlessly progress from initial discovery to preclinical validation.
Our one-stop solution encompasses:
Engineering and Manufacturing
State-of-the-art protocols for extracting high-purity ACExos using techniques such as ultracentrifugation and size exclusion chromatography (SEC).
Advanced multi-omics analyses, including exosomal proteomic detection services, exosomal RNA sequencing services, exosomal lipidomics and metabolomics services, to fully elucidate the bioactive cargo of ACExos and uncover their mechanisms of action.
Custom-designed assays to evaluate cellular uptake, cytotoxicity, and therapeutic efficacy. We support this with services like tumor model construction and biodistribution studies using our exosome labeling technologies.
Sophisticated capabilities for exosome modification, coupled with scalable manufacturing processes that include rigorous stability, purity, and safety evaluations to ensure the highest quality standards.
Collaborate with Us — From Discovery to Application.
Ready-to-Deploy Products
At Creative Biolabs, we leverage our advanced exosome development platform to deliver high-quality Asparagus cochinchinensis–derived exosome (ACExo) products for both research and commercial applications.
Research-Grade ACExos
We provide highly purified, well-characterized ACExos designed to support scientists in academia and industry. Our preparations offer a consistent, reliable source for basic research, target validation, and preclinical studies — helping accelerate discovery while reducing experimental variability and cost.
Cosmetic and Skincare Applications
With growing interest in the dermatological potential of plant-derived exosomes, Creative Biolabs provides custom manufacturing services of ACExos as an advanced bioactive ingredient for skincare and cosmeceutical formulations. We also offer a collaborative evaluation program: clients can submit plant material of interest for preliminary yield assessment. Based on these results, we provide a detailed quotation for customized large-scale production tailored to specific commercial needs.
Inquire About Your Custom Production Project.
FAQs
Q: What fundamentally distinguishes ACExos from exosomes derived from other plants, such as ginger or grapefruit?
A: While all PDExos share structural similarities, their therapeutic uniqueness lies in their specific bioactive cargo, which is a direct reflection of the source plant's biochemistry. ACExos are distinguished by their payload derived from Asparagus cochinchinensis, which includes specific polysaccharides and steroidal saponins known for their potent anti-inflammatory and anti-tumor effects, as demonstrated in the context of hepatocellular carcinoma.
Q: How does Creative Biolabs ensure the consistency and purity of ACExo preparations between batches?
A: We employ a stringent quality control pipeline for every batch. This includes standardized protocols for plant cultivation and harvesting, followed by rigorous purification. Each batch is then subjected to a comprehensive characterization panel—including NTA for size/concentration and TEM for morphology —to ensure it meets our high standards for purity, integrity, and batch-to-batch consistency.
Q: Can ACExos be loaded with custom therapeutic cargo, such as siRNA or small-molecule drugs?
A: Absolutely. ACExos serve as an excellent natural nanocarrier. Our services include the development and optimization of loading protocols (e.g., electroporation) to efficiently encapsulate a wide range of custom payloads, from nucleic acids and proteins to hydrophobic small molecules, transforming the ACExos into a bespoke drug delivery system.
Q: What is the typical exosome yield from Asparagus cochinchinensis?
A: The yield of exosomes can vary depending on several factors, including the specific plant cultivar, growth conditions, and the part of the plant being processed (e.g., root, stem). To provide an accurate assessment, we recommend our initial evaluation service, which quantifies the achievable yield from a specific source material before committing to a large-scale production project.
Q: Beyond the applications discussed, are there other emerging research areas for ACExos?
A: Yes, the field is rapidly evolving. We are actively exploring the potential of ACExos in neurodegenerative diseases, where their ability to cross biological barriers and deliver antioxidant and anti-inflammatory agents could be highly beneficial. Additionally, their role in modulating the gut microbiome is another exciting frontier, potentially leading to novel treatments for metabolic and gastrointestinal disorders.
Start Your ACExo Innovation Journey Today.
Reference
-
Wang, Meng, et al. "Asparagus cochinchinensis: A review of its botany, traditional uses, phytochemistry, pharmacology, and applications." Frontiers in Pharmacology 13 (2022): 1068858. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.3389/fphar.2022.1068858
For Research Use Only. Cannot be used by patients.
Related Services:

 Fig.1 A. cochinchinensis plant morphology.1
Fig.1 A. cochinchinensis plant morphology.1










