Safety Evaluation Service for Candidate Exosome Drug
Overview Services Features FAQs
Overview
The Safety Evaluation of Candidate Exosome Drug Still Faces Some Challenges
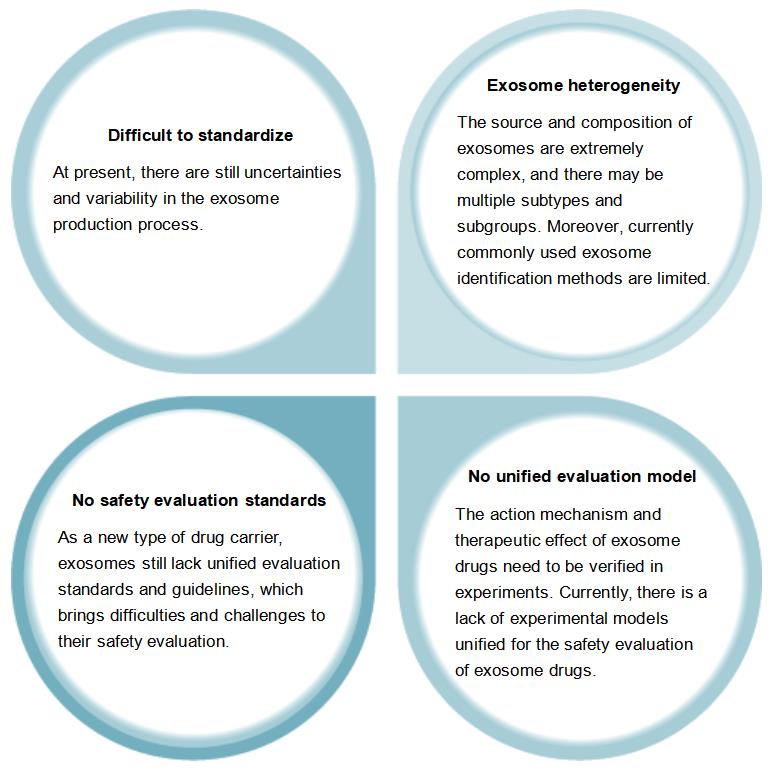
Creative Biolabs is a biotechnology company focusing on biotechnology and drug development, dedicated to helping customers accelerate the development of new drugs. In recent years, exosomes have received extensive attention as a potential means of intercellular communication and signaling. Exosomes have diverse sources and compositions. Different therapeutic effects can be achieved by changing the composition and structure of exosomes. Therefore, exosomes are considered as potential new drug carriers. However, the safety evaluation of exosome drugs still faces some challenges and difficulties.
Services
Comprehensive Safety Evaluation Service for Candidate Exosome Drug at Creative Biolabs
The safety evaluation of exosome drugs needs to comprehensively consider their physiological activity, pharmacokinetics, immunogenicity, toxicology, and other factors. Creative Biolabs provides a complete set of safety evaluation services for candidate exosome drugs, aiming to provide customers with comprehensive, efficient, and reliable exosome drug safety evaluation solutions based on exosome drug safety evaluation solutions based on animal models and cell models.
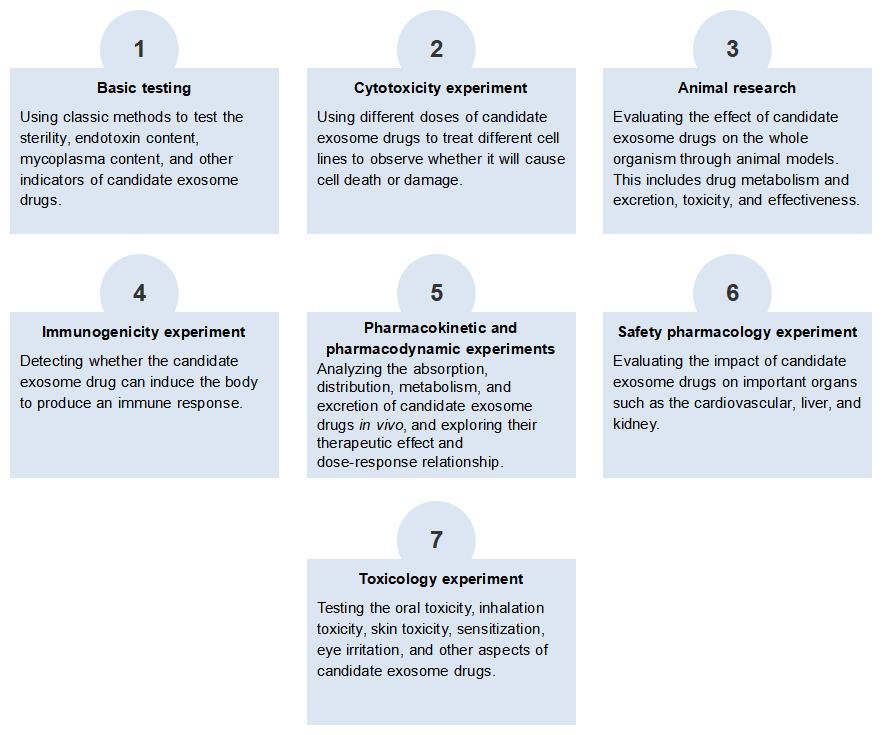
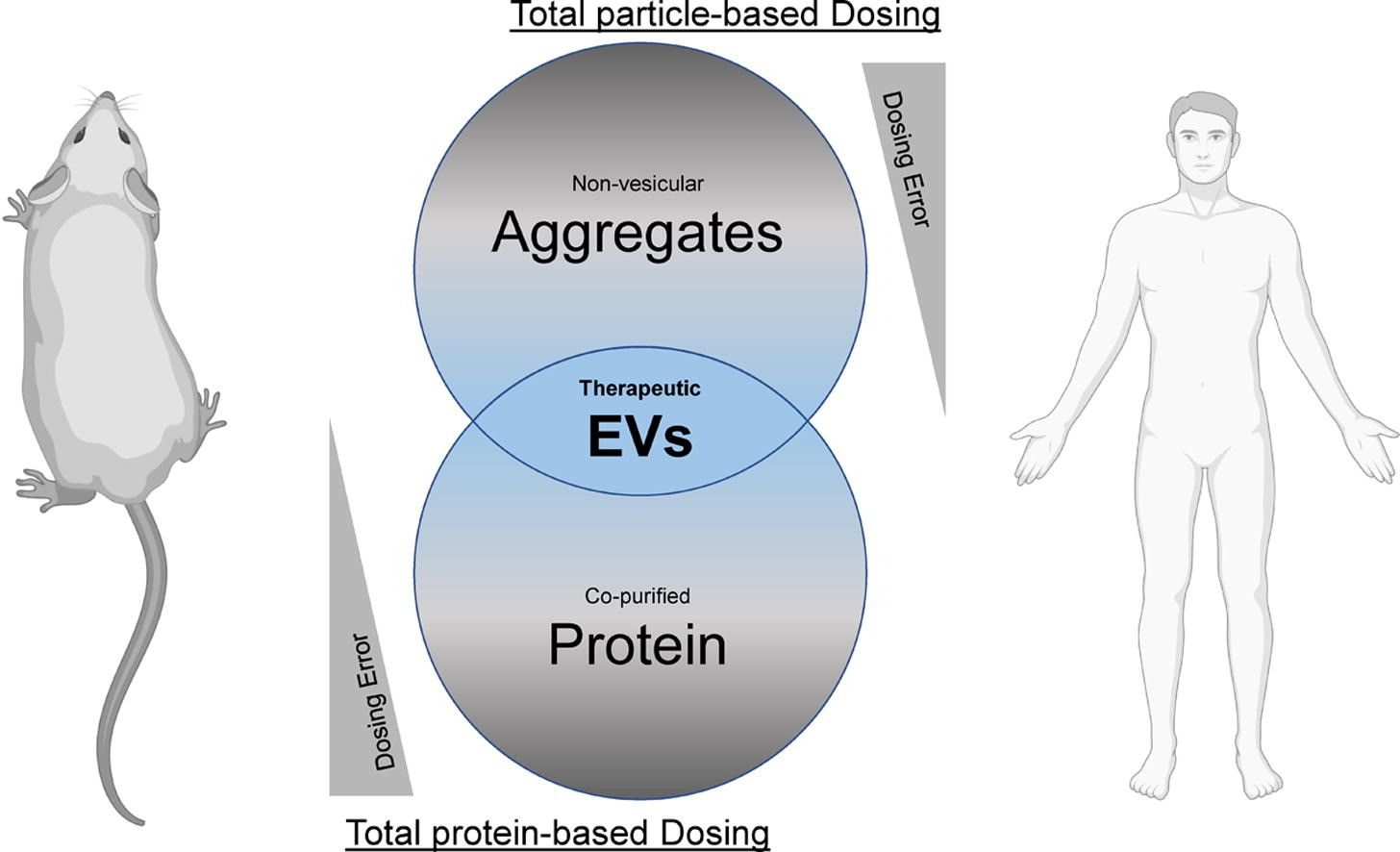 Fig.1 Dose discrepancies depending on the disease model.1,2
Fig.1 Dose discrepancies depending on the disease model.1,2
Features
-
Multiple safety evaluation methods
-
Professional technical team
-
Customized evaluation plans
-
Efficient service process
If you are looking for a reliable biotech company to evaluate the safety of your exosome drug candidates, then Creative Biolabs will be your partner of choice. Please contact us. We conduct various tests in strict accordance with the safety evaluation standards of biological products so that you can refer to the safety and effectiveness of candidate drugs in the research stage.
FAQs
Q: What types of exosome-based drug candidates can be evaluated for safety?
A: We can evaluate the safety of various exosome-based drug candidates, such as adipose-derived mesenchymal stem cells, exosomes surface-modified with targeting peptides, drug-loaded exosomes, and plant exosomes.
Q: Can you evaluate the safety of exosomes intended for specific therapeutic or diagnostic applications?
A: Yes, we can tailor our safety evaluation services to the specific application of your exosome-based drug candidates, whether they are intended for therapeutic, diagnostic, or research purposes. Our assessments will focus on the relevant safety aspects to ensure they are suitable for the intended use.
Q: What is the typical quote for your exosome safety assessment service?
A: The cost of our exosome safety evaluation services varies depending on the specific tests and the scope of the evaluation required. Our technical team provides customized solutions and corresponding quotes based on the project description and expected goals. Please contact us to discuss your project. Our team will send you a detailed proposal and pricing after understanding your overall needs.
Reference
-
Gupta, D.; et al. Dosing extracellular vesicles. Advanced Drug Delivery Reviews. 2021, 178:113961.
For Research Use Only. Cannot be used by patients.
Related Services:



 Fig.1 Dose discrepancies depending on the disease model.1,2
Fig.1 Dose discrepancies depending on the disease model.1,2









