CD47-Expressed Exosome Modification Service
Overview Services Features FAQs
Modifying the surface of exosomes with the transmembrane protein CD47 allows them to interact with the SIRPα receptor on macrophages, protecting the exosomes from phagocytosis and enhancing their accumulation in tumor tissues. Creative Biolabs provides tailored CD47-expressed exosome modification and manufacturing services to enhance the targeted delivery efficiency of exosomes.
Anti-phagocytosis Effect of CD47-SIRPα Pathway
CD47, also known as integrin-associated protein, is a widely expressed transmembrane glycoprotein that serves as a "marker of self" among cellular expression targets. It protects host cells by preventing phagocytosis.
When expressed on host cells, CD47 binds to SIRPα and SIRPγ on immune cells and interacts with integrins, leading to the phosphorylation of ITIM tyrosines. This phosphorylated ITIM recruits and activates the tyrosine phosphatase SHP-1/2 protein, which inhibits the aggregation of myosin at the subsynaptic assembly site of macrophages, sending a "don't eat me" signal. As a result, macrophage phagocytosis is inhibited, protecting cells from being eliminated by the immune system.
CD47 is crucial for maintaining tissue homeostasis, and its absence results in the phagocytosis of old or damaged cells. Therefore, CD47 can be used to modify the surface membrane proteins of exosomes, giving them anti-phagocytic properties.
Modification Strategies of CD47-expressed Exosomes
Exosomes are distinguished from other nanoparticle carriers by the presence of transmembrane proteins on their surface, enhancing endocytosis and content delivery. The engineered modification of CD47-expressing exosomes blocked the formation of macrophage pseudopods and evaded phagocytosis. Combined with multiple interventional treatments as well, it has been applied in several studies of tumor treatment, which exerted the strong permeability and excellent retention effect of exosomes in tumors.
-
One study constructed a CD47 overexpression plasmid and effectively loaded CD47 onto exosomes of donor cells by gene transfection. Such CD47 surface-functionalized exosomes exhibited lower toxicity to the liver and kidney compared to control exosomes.
-
Exosomes secreted by normal fibroblast-like mesenchymal cells were genetically engineered to modify CD47 to enhance the specificity of the loaded shRNA for targeting KRAS in pancreatic cancer.
-
Exosome surface modification of CD47 is compatible with the modification or loading of other functional cargoes. There are studies to develop engineered sEVs with high CD47 expression and c(RGDyC) modification for the delivery of epigenetically regulated proteins targeting gastric cancer. This versatile exosome platform exhibits great potential for intervention in various types of malignancies and is a specific and low-toxicity strategy.
Features
-
Customized Exosome Engineering: Tailor exosomes expressing CD47 to your specific research or therapeutic needs.
-
Enhanced Immune Evasion: CD47-expressed exosomes promote immune evasion, vital for targeted delivery and sustained effects.
-
Precise Cargo Loading: Deliver payloads with precision, ensuring efficient and targeted delivery to desired cells.
-
Versatile Applications: Ideal for drug delivery, immunotherapy, and research applications across diverse fields.
-
Expert Consultation: Leverage our expertise for guidance on experimental design and optimization of CD47-expressed exosomes.
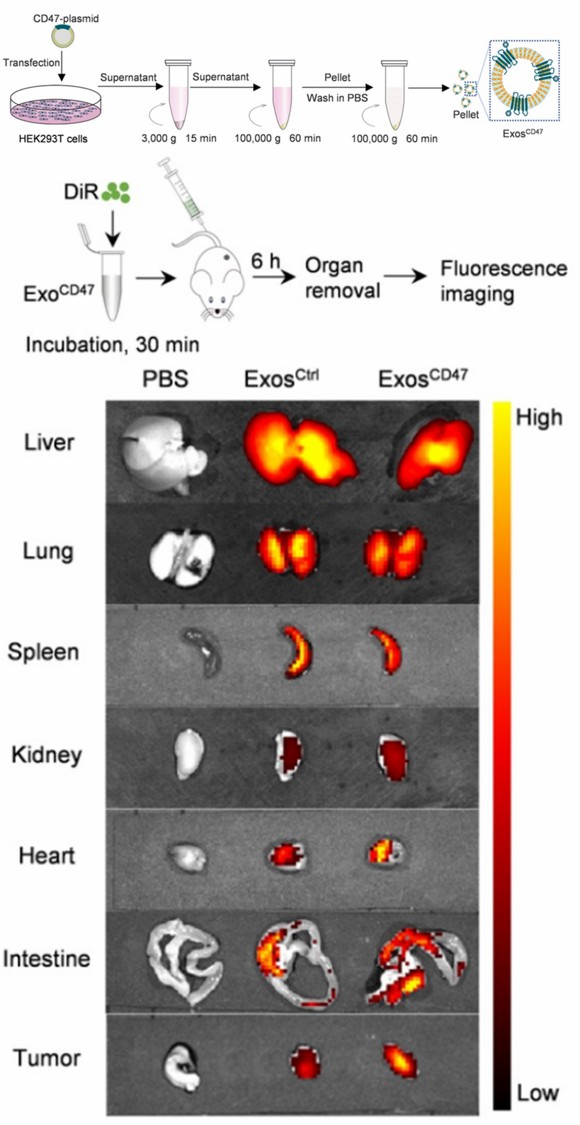 Fig.1 ExosCD47 escape from MPS in vivo.1
Fig.1 ExosCD47 escape from MPS in vivo.1
Most injected nanomaterials are phagocytosed by MPS, preventing them from being delivered to the desired region, with various problems such as poor biodistribution and cationic ligand toxicity in applications. Creative Biolabs has established a comprehensive research platform including exosome engineering modification, cargo loading, labeling, and tracking, enabling it to provide CD47-expressed exosome modification and related probing services. Please contact us with your interest.
FAQs
Q: What is CD47?
A: CD47 is a cell surface protein that plays an important function in immunological control, particularly in phagocytosis.
Q: How can CD47-expressed exosomes benefit my research?
A: By expressing CD47 on exosomes, you can enhance their immune evasion properties, enabling targeted delivery and improved therapeutic outcomes.
Q: Can I customize the cargo loaded into CD47-expressed exosomes?
A: Yes, our service allows precise cargo loading, ensuring the delivery of specific molecules or drugs to target cells.
Q: What applications are CD47-expressed exosomes appropriate for?
A: These exosomes are versatile and find applications in drug delivery, immunotherapy development, and various research endeavors.
Reference
-
Du, Jianbing, et al. "Designer exosomes for targeted and efficient ferroptosis induction in cancer via chemo-photodynamic therapy." Theranostics 11.17 (2021): 8185. Under Open Access license CC BY 4.0. The image was modified by extracting and using only parts of the original image, and by revising the title.
For Research Use Only. Cannot be used by patients.
Related Services:

 Fig.1 ExosCD47 escape from MPS in vivo.1
Fig.1 ExosCD47 escape from MPS in vivo.1









