Cell-derived Exosome-based Delivery Vehicle Feature Summary
Exosomes obtained from commonly produced cell lines capable of carrying a variety of naturally active molecules and acting as a drug delivery platform are significant for their potential therapeutic carrier effects. Creative Biolabs has a long-standing commitment to exosome production, investigation, and application. With pioneering exosome insights, we provide high-quality exosome isolation, purification, and profiling services based on proper exosome research strategies.
Multiple commonly procured cell lines have been extensively expanded and from which exosomes with specific functions have been extracted for drug-delivery vehicle systems. The characteristics of four common types of production cell-derived exosomes and the advances in applications are summarized as follows.
HEK (Human Embryonic Kidney) 293 Cell-Derived Exosomes
HEK293 cells are widely used in biologics production as a mammalian cell line that to provide a production resource. It allows various transgenic approaches to genetically manipulate HEK293 cells to design surface-modified exosomes or to encapsulate functional active cargo during biogenesis. Compared to other cell lines, HEK293 cells exhibit the advantage of higher growth density and faster growth rate and are easily cultured and transfected to meet the scale-up requirements for exosome production. More notably, HEK293 cell-derived exosomes have outstanding immune inertness and are suitable for the delivery of engineered proteins, recombinant viruses, vaccines, and biologics targeting receptors without triggering immune responses and inflammatory damage.
MSCs (Mesenchymal Stem Cells)-Derived Exosomes
MSCs differentiated from stem cells and their exosomes have a strong potential for applications in skin repair, anti-aging, and disease therapy involving the mitigation of damage. Exosomes have been found to inherit high levels of maternally secreted trophic and growth-promoting factors upon release from MSCs and are a new trend in the fields of cardiovascular disease therapy, skin repair, and anti-aging. MSCs-derived exosomes significantly increase cardiomyocyte survival after transplantation, resist cardiomyocyte injury and apoptosis, promote neovascularization and improve cardiac function. Other studies have also demonstrated that MSCs exosomes regulate the secretion of pro-inflammatory cytokines in the skin microenvironment, promote vascularization and collagen deposition in skin defects, as well as regulate the proliferation and differentiation of skin fibroblasts, thus exerting pro-wound healing and inhibiting scar formation. In addition, MSCs are easily isolated from tissues during the cell preparation phase of exosome production and have superior expansion capacity, making them compatible with exosome scale production for both autologous and allogeneic applications.
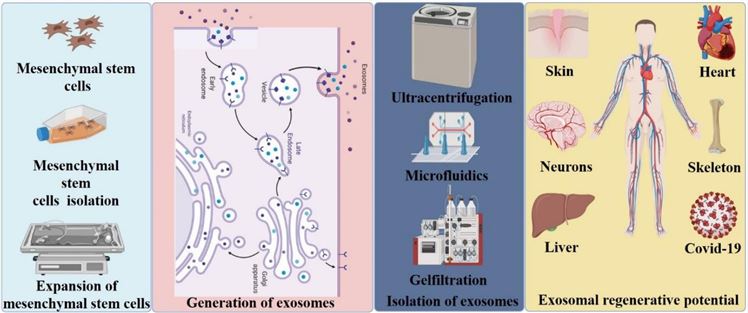 Fig.1 MSC-derived exosome methodology. (Hade, 2021)
Fig.1 MSC-derived exosome methodology. (Hade, 2021)
Immune Cell-Derived Exosomes
As an important source of exosome-producing cells, there are diverse immune cell-derived exosome carriers including DCs (dendritic cells), imDCs (immature dendritic cells), NK cells (natural killer cells), and effector CAR-T cells, which inherit the maternal effector being able to mediate and regulate immune responses. For example, DCs-derived exosomes have the biological function of presenting antigens to T cells and stimulating a robust immune response, expanding the pathway for the development of cell-free nanoscale vaccines that enhance the immune response to cancer. One study with the selection of DCs as the exosome vehicle source induced and boosted the anti-cancer viability of NK cells in a vaccine prepared against non-small cell lung cancer. Meanwhile, given the phenotype of imDCs with lower cross-expression capacity and less co-stimulatory molecule expression, they can confer exosome tolerance characteristics and lower immunogenicity as donor cells. NK cells generate exosomes that mediate the killing effect of NK cells on tumor cells, which contain granulins and perforins as core weapons that exert cytotoxicity upon binding and release into tumor cells. In addition, genetically engineered T cells expressing CAR (chimeric antigen receptor) exert a rapid and durable tumor therapeutic response but involve acute toxicity. Accordingly, effector CAR-T cells have been chosen to produce exosomes carrying CARs on their surface capable of mediating high levels of cytotoxic tumor growth inhibition. CAR exosomes are relatively safe as a therapeutic nano-delivery platform compared to CAR-T cells that do not express programmed cell death protein I.
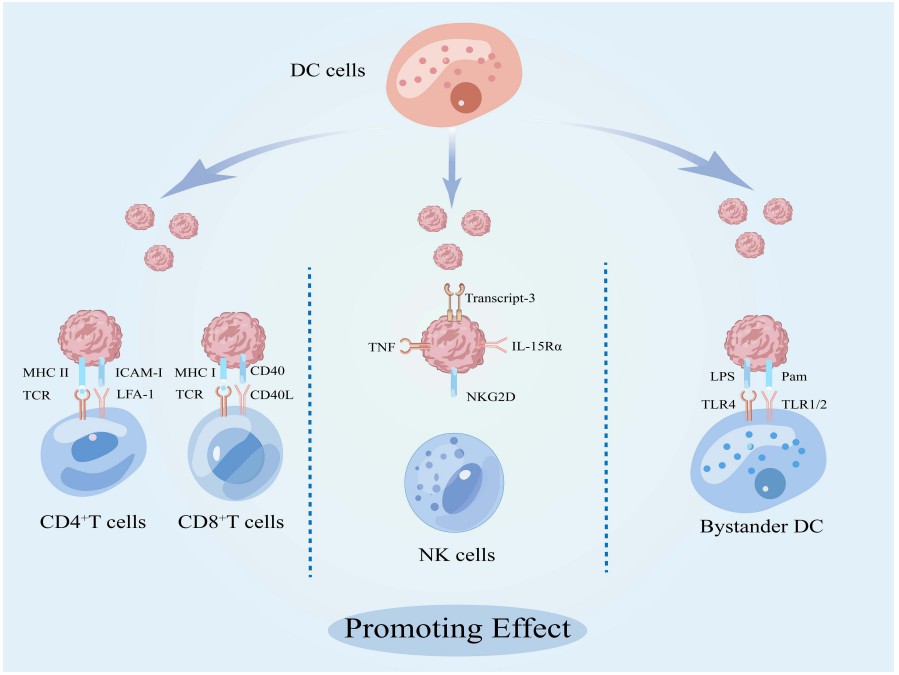 Fig.2 Promoting effect of DC cell-derived exosomes on other immune cells. (Wang & Shi, 2022)
Fig.2 Promoting effect of DC cell-derived exosomes on other immune cells. (Wang & Shi, 2022)
Tumor Cell-Derived Exosomes (TEX)
With the success of immune checkpoint therapies in multiple cancer types, interest in exosome-based tumor immunotherapy has been raised, promising to expand the concept of anti-cancer vaccine development. TEX carry specific antigens that can be utilized as a source of stimuli to induce homotypic tumor immune responses, involving unique homing properties. For example, evidence suggests that TEX acting on dendritic cells of malignant gliomas effectively induce anti-tumor responses against autologous tumor cells. Subsequently, TEX have been found to have the same potential to enhance immune activity in homozygous tumors of various animal models, including colorectal cancer, metastatic skin cancer, and non-small cell lung cancer. However, TEX-mediated immune activation after antigen presentation is also accompanied by the risk of immunosuppression associated with the ligands that they carry, such as proteins and miRNAs. Therefore, an in-depth understanding and systematic evaluation of the endogenous components of TEX and their potential functional mechanisms are desirable to leverage their therapeutic potential.
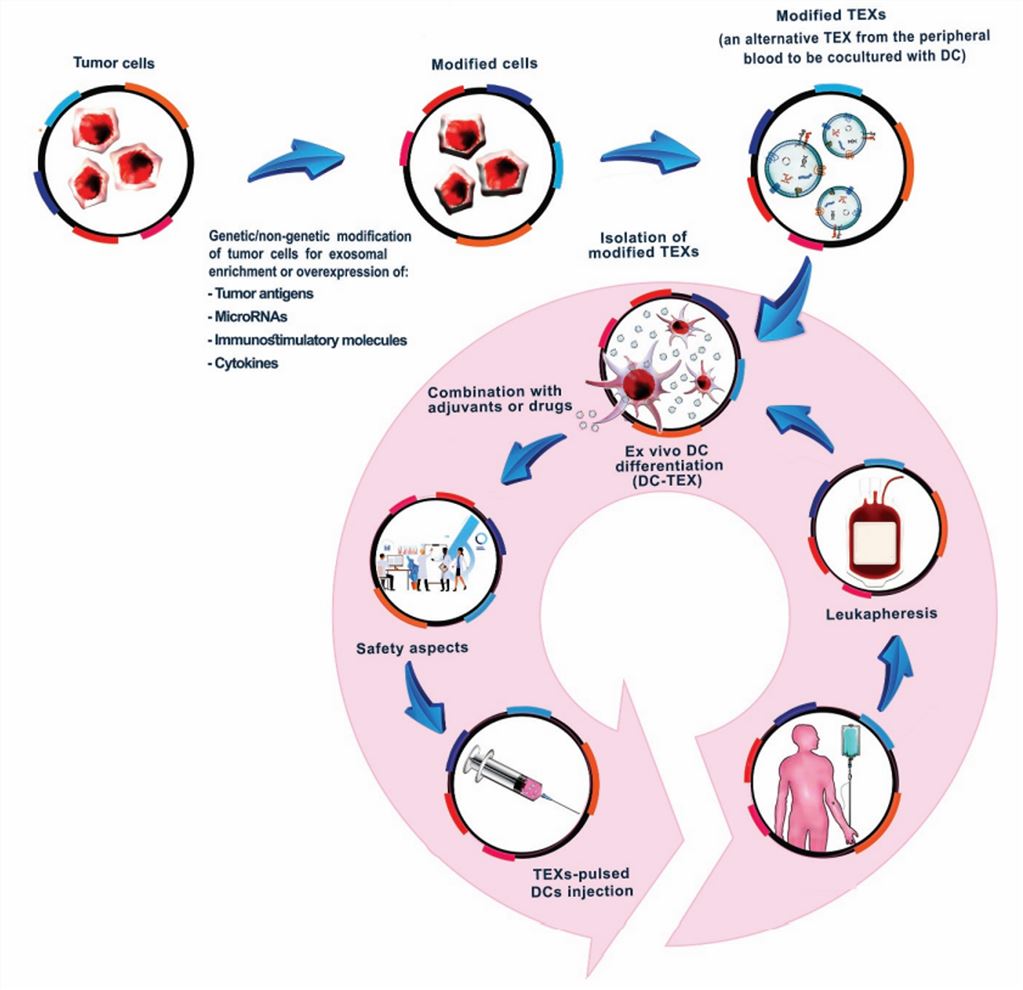 Fig.3 Tumor-derived exosome modulations aiming for increased efficacy of future TEX-based vaccines. (Naseri, 2020)
Fig.3 Tumor-derived exosome modulations aiming for increased efficacy of future TEX-based vaccines. (Naseri, 2020)
Various cell lines have been utilized as a source of exosome production to obtain the exosomes that inherit similar physiological functions from their parental origin as well as to develop potential therapeutic carrier roles. It is a critical aspect to select the appropriate cell type for exosome production to control the exosome endogenous composition and its potential risk-benefit strictly. Depending on the nature of the sample and the research objectives, Creative Biolabs offers customized exosome production and research services by designing appropriate exosome isolation and analysis strategies to meet the client’s needs. Please contact us to discuss your project.
References
-
Hade, M.D.; et al. Mesenchymal stem cell-derived exosomes: applications in regenerative medicine. Cells. 2021, 10(8): 1959.
-
Wang, S.; Shi, Y. Exosomes derived from immune cells: the new role of tumor immune microenvironment and tumor therapy. Int J Nanomedicine. 2022, 17: 6527-6550.
-
Naseri, M.; et al. Tumor-derived exosomes: the next generation of promising cell-free vaccines in cancer immunotherapy. Oncoimmunology. 2020, 9(1): 1779991.
For Research Use Only. Cannot be used by patients.
Related Services:

 Fig.1 MSC-derived exosome methodology. (Hade, 2021)
Fig.1 MSC-derived exosome methodology. (Hade, 2021)
 Fig.2 Promoting effect of DC cell-derived exosomes on other immune cells. (Wang & Shi, 2022)
Fig.2 Promoting effect of DC cell-derived exosomes on other immune cells. (Wang & Shi, 2022)
 Fig.3 Tumor-derived exosome modulations aiming for increased efficacy of future TEX-based vaccines. (Naseri, 2020)
Fig.3 Tumor-derived exosome modulations aiming for increased efficacy of future TEX-based vaccines. (Naseri, 2020)