Fluorescent Dye based Exosome Labeling Service
Overview Services Features FAQs
Overview
Exosomes, as nano-scale particles, need to be labeled to observe the traces of exosomes in vivo and in vitro. For the study of exosome function, it is generally necessary to observe whether the corresponding phenotypic indicators in the recipient cells, tissues, or organs change after the exosomes are taken up by the recipient cells or reach the target tissues and organs. Therefore, exosome labeling is the basis and key step for the study of exosome function. Creative Biolabs has a complete technical platform and rich service experience and can provide a variety of commonly used fluorescent dyes for labeling exosomes, including carbocyanine dyes, PKH dyes, and penetrant dyes.
In the exosome-related articles published so far, most of the exosomes are labeled with lipophilic dyes. The specific labeling principle is that the lipophilic fluorescent dye embeds its aliphatic tails into the phospholipid biomolecules of exosomes in a non-covalent manner, thereby emitting a stable and long-lasting strong fluorescent signal.
Tab. 1 Dyes for Labeling Exosomes
|
Category
|
Dyes
|
Characteristics
|
Mechanism of Action
|
Application
|
|
Lipophilic Carbocyanine Dyes
|
DiL, DiR, DiO, DiD
|
High extinction coefficient, polarity dependence, short excited state lifetime; clean background to avoid interference from autofluorescence in the visible range
|
After entering the exosome membrane, the dyes diffuse and label the entire membrane structure, significantly enhancing fluorescence intensity
|
Labeling cell membranes and other lipid-soluble biological structures
|
|
Lipophilic PKH Dyes
|
PKH-67, PKH-26
|
Low toxicity, low fluorescence background, high fat solubility, strong penetrability, strong and stable fluorescence; PKH-67 has a fluorescent half-life of 10-12 days in vivo, PKH-26 can last up to 100 days or more
|
PKH dye molecules have long aliphatic tails that insert into the phospholipid bilayers while the fluorophores stay outside the exosome membrane and emit strong fluorescence
|
Labeling exosomes
|
|
Penetrating Dyes
|
CFSE, Calcein-AM
|
CFSE is colorless and non-fluorescent itself; Calcein-AM increases hydrophobicity by introducing AM groups
|
CFSE reacts with intracellular amino groups after esterases remove the acetate group, forming a stable fluorescent conjugate; Calcein-AM is cleaved by esterases to form impermeable Calcein, emitting strong green fluorescence
|
Detecting the integrity of exosomes
|
Tab. 2 Excitation and Emission Wavelengths of Commonly Used Fluorescent Dyes
|
Fluorescent dyes
|
Observed Color
|
Marked location
|
Excitation Wavelength
|
Emission Wavelength
|
|
DiO
|
Green
|
Intramembrane
|
484 nm
|
501 nm
|
|
DiL
|
Orange
|
Intramembrane
|
549 nm
|
565 nm
|
|
DiD
|
Red
|
Intramembrane
|
644 nm
|
665 nm
|
|
DiR
|
Deep Red
|
Intramembrane
|
750 nm
|
780 nm
|
|
PKH-67
|
Green
|
Extramembrane
|
484 nm
|
501 nm
|
|
PKH-26
|
Red
|
Extramembrane
|
551 nm
|
567 nm
|
|
CFSE
|
Green
|
Inside
|
494 nm
|
521 nm
|
|
Calcein-AM
|
Green
|
Inside
|
490 nm
|
515 nm
|
Services
The above-mentioned commonly used fluorescent dyes for labeling exosomes can be used for in vivo and in vitro functional studies of exosomes, such as exosome tracking services such as exosome labeling, in vitro cell uptake, and in vivo animal imaging. Creative Biolabs has launched various exosome dye products and exosome tracing services. You can contact us to put forward your needs according to your research purposes. We will make the most suitable plan for you.
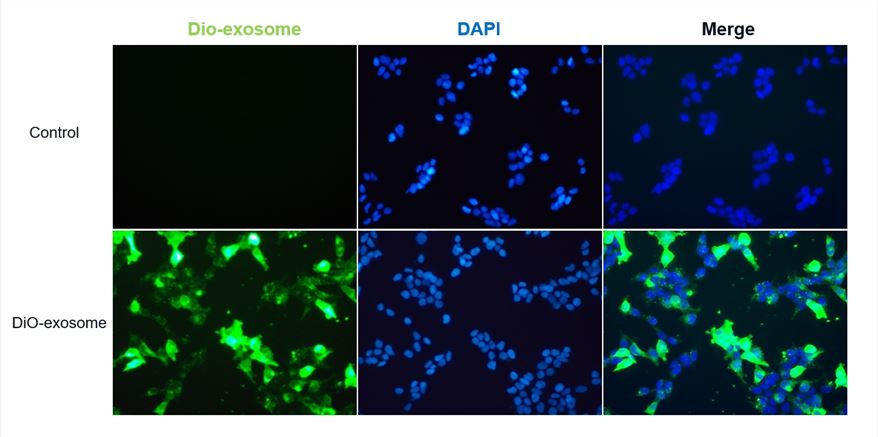 Fig.1 Exosomes labeled using the DiO-Membrane EVs Labeling & Purification Kit (green) (Cat.# ELP-01) were internalized by HEK 293T cells.
Fig.1 Exosomes labeled using the DiO-Membrane EVs Labeling & Purification Kit (green) (Cat.# ELP-01) were internalized by HEK 293T cells.
Features
-
Diverse dye selection
-
Efficient labeling technology
-
High-quality fluorescence imaging
-
Strict experimental procedures
FAQs
Q: How does your technical service ensure the activity of exosomes during the entire labeling process?
A: We use optimized labeling techniques and strict experimental procedures to protect the integrity of the exosomes to the greatest extent possible.
Q: What research applications can labeled exosomes be used for?
A: Labeled exosomes can be used in various research applications, such as flow cytometry, cell uptake experiments, and in vivo and in vitro tracking. Our labeling service is designed to provide reliable tools and data support for your research.
Q: What sample quantity and volume can you handle?
A: We can handle samples of various scales, from small experimental batches to large-scale samples. The required sample volume will depend on the specific experimental design and labeling needs.
Q: What should customers do before submitting samples?
A: We will provide you with detailed sample preparation guidelines, including specific requirements for sample collection, storage, and transportation. Ensuring sample quality is crucial for achieving optimal labeling results.
Q: Are labeled exosomes suitable for in vivo experiments?
A: Yes, labeled exosomes can be used for in vivo experiments, such as exosome tracking and distribution studies in animal models. To avoid interference from tissue autofluorescence, it is recommended to use red dyes, such as DiD, DiR, and PKH-26.
For Research Use Only. Cannot be used by patients.
Related Services:

 Fig.1 Exosomes labeled using the DiO-Membrane EVs Labeling & Purification Kit (green) (Cat.# ELP-01) were internalized by HEK 293T cells.
Fig.1 Exosomes labeled using the DiO-Membrane EVs Labeling & Purification Kit (green) (Cat.# ELP-01) were internalized by HEK 293T cells.









