Purity Evaluation Service for Exosome Product
Overview Services Features FAQs
Overview
The Importance of Exosome Product Purity Evaluation


The purity evaluation of exosome products is crucial to scientific research and future medical applications and can provide a strong basis for the accuracy of experimental results, product safety, and effectiveness. In order to meet customer needs and ensure product safety and compliance, Creative Biolabs provides a full range of technical services to help evaluate the purity of exosome products.
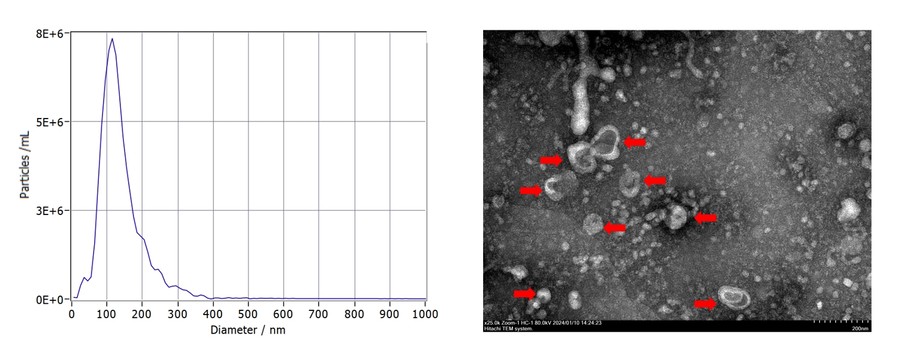 Fig.1 Particle size evaluation and morphological evaluation.
Fig.1 Particle size evaluation and morphological evaluation.
Services
The Purity Evaluation System of Exosome Products in Creative Biolabs
For the comprehensive evaluation of the purity of exosome products, Creative Biolabs can use the following technologies for analysis and evaluation. Most importantly, we will combine the results of different technologies to confirm, complement and verify each other to ensure that the final evaluation results are accurate and reliable.
|
Evaluation technology
|
Evaluation detail
|
|
Morphological evaluation
|
The shape, size, and quantity of exosomes can be observed by electron microscopy. Under the electron microscope, the background of high-purity exosome products is clean, and the particle size is relatively uniform.
|
|
Particle size evaluation
|
Nanoparticle tracking analysis can be used to obtain the number distribution map of different particle sizes. The particle size distribution of high-purity exosome products is concentrated in the range of 30-150nm. The ratio of particles of the correct size can be used as an indicator of purity.
|
|
Purity calculation
|
Bicinchoninic acid assay can be used to quantify the protein concentration of exosome products. The ratio of the number of particles to the amount of protein can also be used as an indicator of purity.
|
|
Chromatographic analysis
|
Various substances in the sample can be separated and quantified by using high-performance liquid chromatography. By calculating the peak area or peak height ratio of each component, the degree of purity of the exosome product can be determined.
|
|
Positive marker evaluation
|
Western Blot (WB), enzyme-linked immunosorbent assay (ELISA), mass spectrometry, and other methods can be used to detect the type and expression abundance of marker proteins in exosomes.
|
|
Negative marker evaluation
|
WB, ELISA, quantitative polymerase chain reaction (qPCR), mass spectrometry, and other methods can be used to detect proteins or nucleic acids that should be expressed in donor cells or biological fluids but should not be expressed in exosomes.
|
|
Molecular spectrum evaluation
|
The purity of exosomes is evaluated by detecting the types and contents of nucleic acids, proteins, lipids and metabolites in exosome products by mass spectrometry.
|
|
Biological activity evaluation
|
Using techniques such as cell culture and mouse models, the safety and effectiveness of exosome products can be evaluated.
|
|
Other possible evaluation methods
|
Features
-
Combination of multiple advanced detection techniques
-
High sensitivity and high precision
-
Professional technical support team
-
Meeting various research and application needs
Creative Biolabs provides exosome product purity evaluation services to help customers optimize the exosome purification process and improve development efficiency and reliability. If you have this demand, please contact us.
FAQs
Q: Why can the ratio of particle count measured by NTA to protein amount measured by BCA be used to evaluate the purity of exosomes?
A: Exosome products may contain various impurities, such as cell debris, lipids, and proteins. The particle count measured by NTA (Nanoparticle Tracking Analysis) reflects the number of exosomes in the sample, while the protein amount measured by BCA (Bicinchoninic Acid Assay) reflects the total protein content in the sample. A higher ratio of particle count to protein amount indicates that each exosome particle carries fewer non-exosomal proteins, resulting in a higher purity of the sample.
Q: Can your company provide customized purity evaluation solutions?
A: Of course, our technical team can tailor purity assessment plans and quotations for customers based on their description of exosome samples and other requirements. Whether it is special detection requirements or specific project goals, we can tailor solutions to meet your needs.
Q: How does your service ensure the rigor and accuracy of purity assessment data?
A: We strictly follow Standard Operating Procedures (SOPs) for each step of the detection process and employ multiple methods for result verification.
For Research Use Only. Cannot be used by patients.
Related Services:



 Fig.1 Particle size evaluation and morphological evaluation.
Fig.1 Particle size evaluation and morphological evaluation.









