All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Cellular immunotherapy holds promise for cancer treatment and has the potential to perform synergy with surgery, chemotherapy, and radiotherapy. Cytokine-induced killer (CIK) cells as a new approach of adoptive cellular immunotherapy, have further promoted the immunotherapy process. CIK cells are a heterogeneous subpopulation of polyclonal T cells possessing natural killer (NK) and T-cell properties, which present potent non-major histocompatibility complex-restricted cytotoxicity activity targeting a variety of tumor types. CIK cells can be genetically modified to express CARs with relatively low safety which serves as a promising approach for cancer immunotherapy.
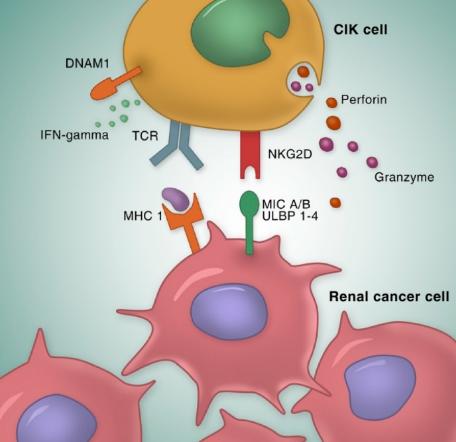 Fig.1 Schematic of CIK cell anti-tumor activity.1
Fig.1 Schematic of CIK cell anti-tumor activity.1
Characteristics of CIK cells
CIK cells are isolated from peripheral blood mononuclear cells (PBMC) under the presence of special cytokines in vitro. Generally, CIK cells are a heterogeneous group of cells characterized by CD3+ T cells also with a CD56+ NK cell phenotype, with a high proliferative rate and potent lytic efficacy against a variety of cancer types, especially some solid tumors. The cytotoxicity of CIK cells relies on the action of natural killer group 2 member D (NKG2D) and its ligand on tumor cells, as well as the progress of perforin-mediated pathways.
CIK cells have been considered as an alternative immune cell source for CAR modification with many features:
CAR-CIK Therapy Possessing Numerous Advantages
Compared to other allogeneic CAR-Ts, CAR-CIK cells show a lot of features for cancer immunotherapy as follows:
CAR-CIK Cells at Creative Biolabs
With years of expertise and experience in immunotherapy discovery and development, Creative Biolabs is dedicated to providing comprehensive antigen-targeting CAR-CIK cells to speed up global customers' programs. Leveraged by a one-stop CellRapeutics™ platform, our service includes CAR designing, vector construction, and CAR-CIK Cells screening process with strict quality control. Meanwhile, we are committed to customizing special CAR products according to the customer's special needs. Please don't hesitate to contact us or inquire about an appropriate product or special solution for you.
Reference
Associated Antigen Target
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION