Introduction of CD3
CD3, comprising four distinct polypeptide chains (γ, δ, ε, and ζ), forms a complex that associates with the T cell receptor (TCR) on the surface of T cells. Playing a pivotal role in TCR signaling, CD3 influences T cell activation, differentiation, and effector functions. Upon antigen recognition by the TCR, CD3 transmits intracellular signals through its immunoreceptor tyrosine-based activation motifs (ITAMs), phosphorylated by Src family kinases. CD3 also recruits and activates various signaling molecules, including ZAP70, PLCγ1, Ca2+ flux, NFAT, NF-κB, AP-1, MAPKs, and the PI3K/Akt pathways. This activation cascade leads to T cell proliferation, cytokine production, cytolytic activity, and memory formation. CD3 emerges as a potential target for cancer immunotherapy, particularly for bispecific antibodies (BsAbs), which crosslink CD3 with tumor-associated antigens (TAAs) to induce TCR-independent T cell-mediated cytotoxicity. Numerous BsAbs targeting CD3 and diverse TAAs are undergoing development and clinical trials for various cancer types.
Introduction of GD2
GD2, a disialoganglioside within the glycosphingolipid family, comprises a ceramide backbone and a complex oligosaccharide chain. Synthesized mainly in the Golgi apparatus, GD2 is enriched in the outer leaflet of the plasma membrane. GD2 serves various biological functions, influencing cell adhesion, signal transduction, differentiation, and migration. It interacts with multiple receptors and ligands, including siglecs, integrins, galectins, and antibodies, thereby modulating the activity of cSrc, FAK, RhoA, Rac1, Cdc42, ERK1/2, JNK, p38 MAPK, AKT/mTOR, and STAT3 pathways. These interactions affect tumor cell survival, growth, invasion, angiogenesis, apoptosis, autophagy, and immune evasion. GD2, a tumor-associated antigen (TAA), is highly expressed in neuroectodermal tumors like neuroblastoma, melanoma, and glioma but has limited expression in normal tissues. GD2 emerges as a promising target for cancer immunotherapy, especially for BsAbs capable of binding GD2 on tumor cells and CD3 on T cells, inducing T cell-mediated cytotoxicity. Several BsAbs targeting GD2 and CD3 are undergoing development and clinical trials, particularly for neuroectodermal tumors.
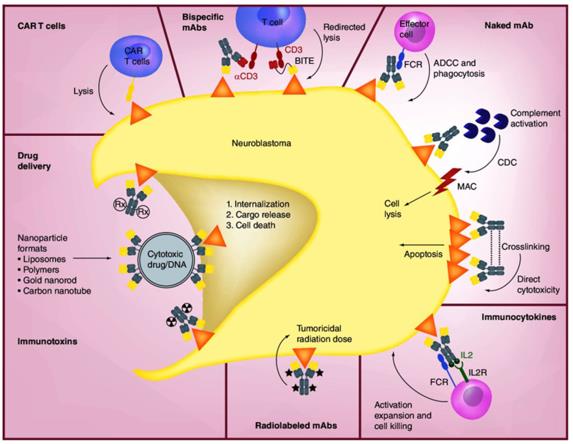 Fig.1 Mechanisms of Anti-GD2 mAbs and Other Therapeutic Approaches (Nazha B, 2020)
Fig.1 Mechanisms of Anti-GD2 mAbs and Other Therapeutic Approaches (Nazha B, 2020)
Signaling Pathways Involved in Bispecific Antibodies Targeting CD3 and GD2
BsAbs designed to target CD3 and GD2 demonstrate the ability to activate or inhibit various signaling pathways in both T cells and tumor cells, leading to diverse biological outcomes. On one hand, BsAbs can facilitate the crosslinking of CD3 on T cells with GD2 on tumor cells, initiating TCR-independent T cell activation and cytotoxicity. This intricate process involves the phosphorylation of CD3's immunoreceptor tyrosine-based activation motifs (ITAMs) by Src family kinases, along with the recruitment and activation of downstream signaling molecules, such as ZAP70, PLCγ1, Ca2+ flux, NFAT, NF-κB, AP-1, MAPKs, and PI3K/Akt pathways. These pathways orchestrate T cell functions, including proliferation, cytokine production, cytolytic activity, and memory formation. Conversely, BsAbs can also bind GD2 on tumor cells, influencing several signaling pathways that impact tumor cell survival, growth, invasion, angiogenesis, apoptosis, autophagy, and immune evasion. These pathways include cSrc, FAK, RhoA, Rac1, Cdc42, ERK1/2, JNK, p38 MAPK, AKT/mTOR, and STAT3. The delicate balance between these signaling pathways plays a pivotal role in determining the efficacy and safety of BsAbs targeting CD3 and GD2 for cancer immunotherapy.
Clinic Status of Bispecific Antibodies Targeting CD3 and GD2
The clinical landscape for BsAbs targeting CD3 and GD2 is rapidly advancing, with several candidates progressing through different developmental stages. Presently, the FDA has approved only one BsAb targeting CD3 and GD2 for clinical use. However, various other BsAbs with this dual targeting approach are actively undergoing clinical development by different entities, employing diverse formats and platforms. One notable example is huGD2-BsAb, a novel BsAb capable of recognizing and binding two distinct antigens simultaneously. This innovative antibody targets GD2 and CD3, surface molecules found on tumor cells and T cells of solid tumors, such as neuroblastoma. The mechanism of action for huGD2-BsAb involves connecting T cells with tumor cells, activating T cell killing functions and eliminating tumor cells. Developed by Y-mAbs Therapeutics, huGD2-BsAb is currently in late-stage preclinical research. Promisingly, it holds potential for treating GD2-positive solid tumors in both adults and children that have proven refractory to other treatment modalities.
Table 1. Summary of huGD2-BsAb
|
Name
|
huGD2-BsAb
|
|
Developer
|
Y-mAbs Therapeutics, Inc.
|
|
Target
|
GD2 and CD3
|
|
Mechanism
|
Engages T cells to kill GD2-expressing tumor cells
|
|
Clinical stage
|
I/II
|
|
NCT number
|
NCT03860207
|
|
Potential indications
|
Neuroblastoma, melanoma, osteosarcoma, small cell lung cancer, etc.
|
References
-
Cheung NV, et al. Targeting CD3 with bispecific antibodies for cancer therapy. Cancer Treat Res Commun. 2020;23:100168.
-
Sait S, et al. Anti-GD2 immunotherapy for neuroblastoma. Expert Rev Anticancer Ther. 2017 Oct;17(10):889-904.
-
Nazha B, et al. Disialoganglioside GD2 Expression in Solid Tumors and Role as a Target for Cancer Therapy. Front Oncol. 2020 Jul 7;10:1000.
-
Yankelevich M, et al. Anti-CD3 × anti-GD2 bispecific antibody redirects T-cell cytolytic activity to neuroblastoma targets. Pediatr Blood Cancer. 2012 Dec 15;59(7):1198-205.
-
Nakajima M, et al. Potent antitumor effect of T cells armed with anti-GD2 bispecific antibody. Pediatr Blood Cancer. 2021 Jul;68(7):e28971.
-
Arendt AM, et al. Targeting GD2 after allogeneic SCT: effector cell composition defines the optimal use of ch14.18 and the bispecific antibody construct NG-CU (GD2-CD3). Cancer Immunol Immunother. 2023 Nov;72(11):3813-3824.
-
Cheng M, et al. Structural design of disialoganglioside GD2 and CD3-bispecific antibodies to redirect T cells for tumor therapy. Int J Cancer. 2015 Jan 15;136(2):476-86.
-
Zirngibl F, et al. GD2-directed bispecific trifunctional antibody outperforms dinutuximab beta in a murine model for aggressive metastasized neuroblastoma. J Immunother Cancer. 2021 Jul;9(7):e002923.
-
Cheng M, et al. Successful engineering of a highly potent single-chain variable-fragment (scFv) bispecific antibody to target disialoganglioside (GD2) positive tumors. Oncoimmunology. 2016 May 5;5(6):e1168557.
-
Lode HN, et al. Bispecific antibodies targeting CD3 and GD2 for the treatment of neuroblastoma and other GD2-positive tumors. Antibodies (Basel). 2020 Dec 18;9(4):80.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY


 Fig.1 Mechanisms of Anti-GD2 mAbs and Other Therapeutic Approaches (Nazha B, 2020)
Fig.1 Mechanisms of Anti-GD2 mAbs and Other Therapeutic Approaches (Nazha B, 2020)