Exosome-Associated Adeno-Associated Virus (AAV) Vector Production Service
Overview Services Features FAQs
The basic principle of gene therapy is introducing exogenous genetic material into cells to modify, replace or regulate specific gene functions. Therefore, viral vectors represent the most promising and useful therapeutic vectors. Although virus production has been widely used in primary and applied research, new methods are still needed to improve quantity, quality, efficacy and safety, to narrow the gap from research results to therapeutic products. Creative Biolabs offers thoughtful exosome-associated AAV production services for customers worldwide.
AAV and Exo-AAV Vectors Development
Among the viral vectors, the AAV vector is one of the front runners in human gene therapy because of its good safety profile in clinical trials. They have demonstrated superior gene expression capabilities in a variety of human tissues, big animal models, non-human primates, and rodents. The powerful and stable gene transfer imparted by AAV to non-dividing cells is attributed to the protein capsid of the virus and the episomal configuration of its DNA in the nucleus. The tropism of AAV differs significantly between its serotypes, and developments in self-complementary DNA and improved capsid technology have increased these vectors' effectiveness even further.
One of the primary obstacles impeding their extensive application is the expense, labor, expertise, and lengthy purification procedure of AAV. According to recent research, exosomes can load AAV (exo-AAV) when the vector is separated from the producer cells' conditioned medium. Moreover, compared to regular AAV, exo-AAV is more resistant to neutralizing anti-AAV antibodies. Compared with the classical purification scheme of iodixanol gradients of AAV, the purification of exo-AAV is simpler. The current purification scheme for exo-AAV isolation relies on the centrifugation step, separating the cell culture supernatant containing exo-AAVs from the producer cells, and then enriching the exosomes containing AAVs.
Our Services
-
AAV Vector Constructs: Our team designs and constructs customized AAV vectors tailored to your research needs. We ensure high efficiency and precision in vector construction, incorporating the desired genetic elements to achieve optimal expression and functionality.
-
Exo-AAV Vector Preparation: Utilizing cutting-edge technology, we produce exosome-associated AAV (exo-AAV) vectors. Our preparation process enhances the delivery efficiency and reduces immunogenicity, making it suitable for various therapeutic and research applications.
-
Characterization of exo-AAV: We provide thorough characterization services for exo-AAV vectors, including particle size analysis, zeta potential measurement, and quantitative PCR. Our detailed characterization ensures the quality and consistency of the exo-AAV preparations, allowing for an accurate assessment of their efficacy and safety in downstream applications.
Features
-
High-Quality Exosome-Associated AAV Vectors: Our service ensures the production of top-tier exosome-associated AAV vectors, meeting stringent quality standards.
-
Expertise in AAV Vector Technology: With a team of seasoned professionals, we leverage cutting-edge AAV vector technology to deliver exceptional results.
-
Strict Quality Control: The safety, potency, and purity of the exosome-associated AAV vectors we create are guaranteed by our strict quality control procedures.
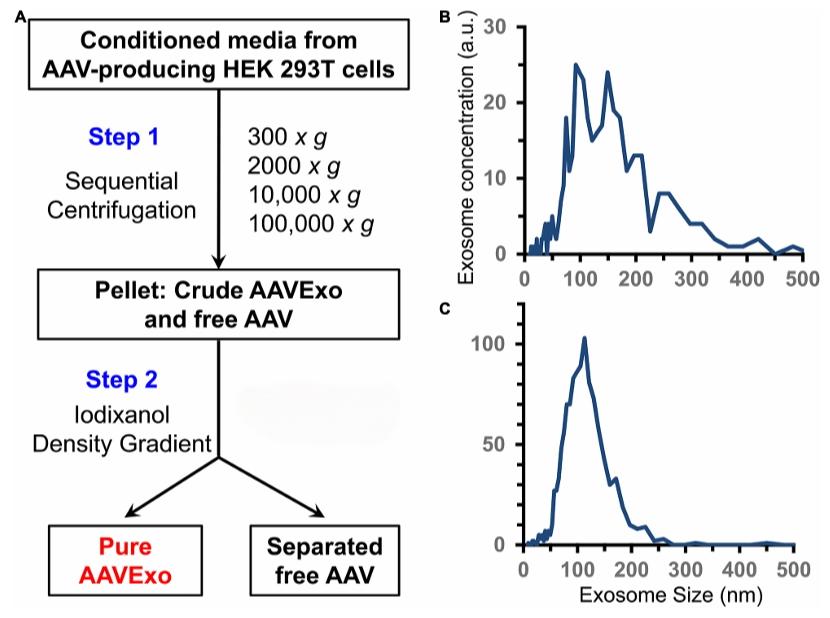 Fig. 1 Exosomes are released and extracted from the AAV-transfected cell conditioned media.1
Fig. 1 Exosomes are released and extracted from the AAV-transfected cell conditioned media.1
In conclusion, exo-AAV appears to target cell types similar manner to conventionally produced AAV particles. Creative Biolabs provides exo-AAV production services that assist clients in increasing transduction efficiency, expanding the use of AAV in target cells and hard-to-reach areas and are more resistant to inactivation caused by anti-AAV antibodies. Please contact us to inquire about a solution.
FAQ
Q: What are exosome-associated AAV vectors?
A: Exosome-associated AAV vectors are a novel delivery system where AAV vectors are encapsulated within exosomes. This approach enhances the efficiency and specificity of gene delivery by leveraging the natural properties of exosomes.
Q: What advantages do exosome-associated AAV vectors offer?
A: Exosome-associated AAV vectors offer several advantages:
-
Enhanced Delivery Efficiency: Improved transduction efficiency due to better cell uptake and reduced immune response.
-
Targeted Delivery: Increased specificity for target tissues or cells.
-
Reduced Immunogenicity: Lower likelihood of being neutralized by pre-existing antibodies compared to traditional AAV vectors.
Q: What controls are in place for quality assurance?
A: We employ optional quality control measures, including:
-
Quantification of AAV vector genome copies
-
Exosome characterization (size, purity, concentration)
-
Biological activity assays
-
Endotoxin and sterility testing
Q: Can I customize the AAV vector for my specific needs?
A: Yes, we offer customization options for the AAV vector, including specific serotypes, promoters, and transgenes to suit your research or therapeutic needs.
Reference
-
Liu, Bin, et al. "AAV-containing exosomes as a novel vector for improved gene delivery to lung cancer cells." Frontiers in Cell and Developmental Biology 9 (2021): 707607. Under Open Access license CC BY 4.0. The image was modified by revising the title.
For Research Use Only. Cannot be used by patients.
Related Services:

 Fig. 1 Exosomes are released and extracted from the AAV-transfected cell conditioned media.1
Fig. 1 Exosomes are released and extracted from the AAV-transfected cell conditioned media.1









