Immune cell function is tightly regulated by two types of receptors: while a costimulatory receptor is activated in response to a foreign antigen, the co-suppressor receptor prevents excessive-immune activation, tissue damage and autoimmunity by inhibiting signaling. Both immunological checkpoints are critical for controlling immune cell activity and can be modulated by blocking or activating antibodies.
Antagonist antibodies that target immune checkpoint co-repressor receptors can reverse the immune resistance of some tumors, and many of these drugs have been approved for the treatment of cancer. Creative Biolabs' expert team is dedicated to the study of key considerations and potential pitfalls in the design and development of antagonist antibodies, which enables us to develop highlighted antagonist antibodies for the clinical development of cancer therapy for our clients.
Lung cancer is the leading cause of cancer-related deaths worldwide, killing more than 1.5 million people each year. Lung cancer is mainly divided into the following two categories: non-small cell lung cancer (NSCLC) (including lung adenocarcinoma, squamous cell carcinoma) and large cell carcinoma histology subtype. Antagonist treatment strategies against immune checkpoints have been shown to have significant anti-tumor responses. The US Food and Drug Administration has approved anti-PD-1 / PD-L1 antagonist antibodies for the treatment of advanced (metastatic) NSCLC, which have been shown great anti-tumor efficacy in lung cancer.
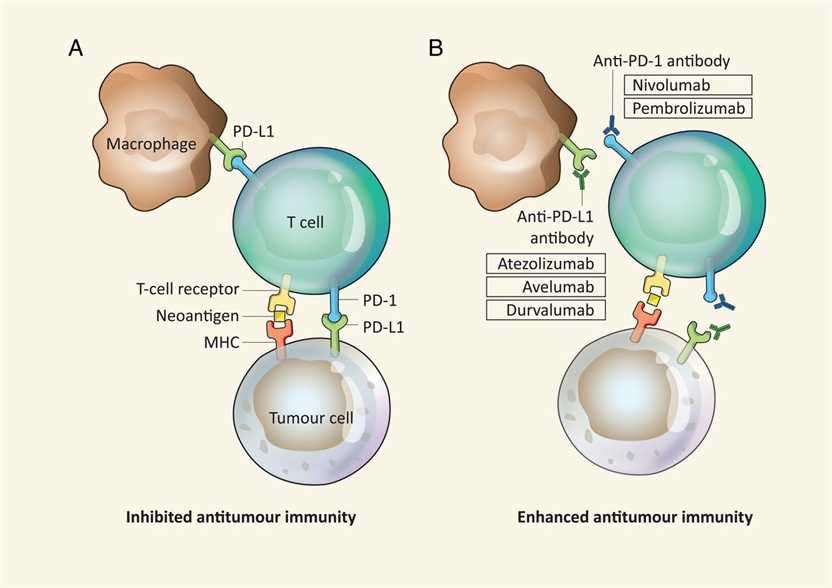 Fig.1 Checkpoint blockade in development in NSCLC. (Manegold, 2016)
Fig.1 Checkpoint blockade in development in NSCLC. (Manegold, 2016)
More and more pharmaceutical companies are joining the highly promising field of lung cancer treatment and doing their utmost to develop antagonist antibodies that target lung cancer. The pioneer is targeting the co-suppressor receptor PD-1 or its major ligand, and four antibodies are currently in the late development of NSCLC. Creative Biolabs has decades of experience in antibody development and is committed to combining our expertise and your expertise to achieve success. Based on the latest clinical research data, we offer our clients the following highlight target (including but not limited to) antagonist antibody design and development services:
| B-RAF | EGFR | PD-1 | PD-L1 | CTLA4 |
| ALK | ROS1 | VEGF | Galectin-3 | Adenosine A2AR |
If you already have a defined target that you are interested in, we also provide end-to-end services for your antagonistic antibody engineering, from antagonistic antibody screening to antagonistic antibody characterization.
If you have specific requirements for the development of antagonist antibodies, our custom antagonisitc antibody development services will provide a solution that will satisfy you.
For additional details for antagonistic antibody services, please for free to contact us.
Reference
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.