Introduction Services Service Workflow Applications Service Highlights Q&A
Creative Biolabs provides a complete platform for trispecific antibody development. Our expert team offers end-to-end services, from target design to manufacturing, ensuring project success. We offer flexible strategic options to meet your project's needs.
Introduction of Trispecific Antibodies
Trispecific antibodies (TsAbs) represent a significant leap forward in targeted therapy, extending beyond the capabilities of traditional monoclonal and bispecific antibodies. Engineered to simultaneously bind three distinct targets, these molecules can orchestrate a more comprehensive and potent therapeutic response. By engaging multiple targets—such as two different tumor antigens and a T-cell engager, or an immune cell with a co-stimulatory receptor and a tumor-associated antigen—TsAbs are designed to overcome key limitations of current therapies. This includes addressing antigen heterogeneity, mitigating immune escape, and enhancing immune cell activation and proliferation in the tumor microenvironment.
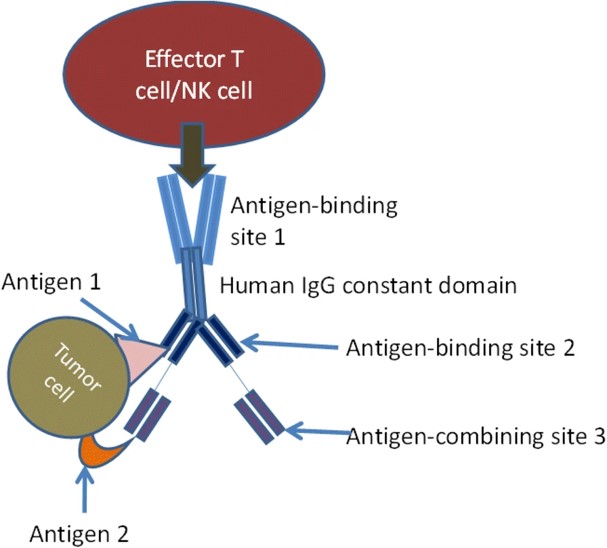 Fig.1 Schematic representation of TsAb structure.1,3
Fig.1 Schematic representation of TsAb structure.1,3
Trispecific Antibody Development Services at Creative Biolabs
Our TsAb development services are built on a modular, integrated platform designed to navigate the complexities of these molecules with maximum efficiency. This strategy allows us to rapidly design, screen, and optimize candidates, streamlining the entire development lifecycle from initial concept to lead optimization. We provide a comprehensive suite of services, starting with our core development expertise and culminating in a full range of analytical support.
Core Development
We handle the foundational steps, including target selection, antibody engineering, and cell line development. Our team leverages deep expertise in protein design to ensure optimal molecular configurations from the outset.
Our services cover various TsAb formats, including but not limited to the following:
-
IgG-like Formats: These molecules contain a full Fc domain, which provides a long serum half-life and allows for standard IgG purification methods. They are suitable for applications where extended circulation is critical.
-
Fc-less Formats: These smaller molecules lack the Fc domain, which enhances tumor penetration and reduces the risk of Fc-mediated off-target toxicity. They are ideal for solid tumor applications where fast clearance is acceptable.
Bispecific T-cell engagers (Bi-TCEs) with a third arm: We can engineer a third binding domain onto an existing Bi-TCE format to enhance T-cell activation or improve tumor targeting. This approach is highly effective for applications where direct immune cell engagement is the primary mechanism of action.
Analytical Services
Our advanced analytical capabilities ensure the quality and performance of your therapeutic. We perform detailed binding analysis, expression optimization, and stability testing to confirm purity and functional integrity.
Safety and Efficacy
We prioritize a favorable safety profile and robust function. Our services include rigorous immunogenicity prediction to mitigate risks and advanced bioassay development to confirm biological activity in relevant systems.
Service Workflow of Trispecific Antibody Development Services
Our service workflow is a systematic, step-by-step process designed for clarity and efficiency.
-
Initial Consultation: We begin with a detailed discussion to understand your specific project goals, requirements, and desired outcomes.
-
Starting Materials: You provide us with the necessary starting materials, which may include target genes, antibody sequences, or other proprietary information.
-
Molecular Cloning & Engineering: Our team performs the molecular cloning and engineering of your TsAb. We use our expertise to create the optimal molecular configuration.
-
Expression & Purification: We express the engineered antibody in our proprietary cell lines and then perform rigorous purification to obtain the final product.
-
Quality Control & Analysis: Throughout the process, we conduct extensive analytical services, including binding analysis and stability testing, to ensure the product meets our high standards.
-
Final Delivery: The project concludes with the delivery of the engineered TsAb, comprehensive data reports, and a detailed intellectual property package. We ensure a seamless transition for your clinical development or research applications.
Applications of Trispecific Antibodies
Cancer Immunotherapy
The primary application of TsAbs is in oncology. They are designed to act as immune cell engagers, linking immune effector cells (like T-cells and NK cells) to cancer cells. This enhances targeted tumor destruction. By simultaneously targeting multiple tumor antigens, they can overcome tumor heterogeneity and antigen escape. Examples include molecules targeting CD3, CD19, and CD20 for lymphoma, or CD3, BCMA, and CD38 for multiple myeloma.
Autoimmune and Inflammatory Diseases
Beyond cancer, TsAbs hold immense promise for treating autoimmune and inflammatory conditions. By simultaneously engaging different immune receptors, they can modulate immune responses more precisely than conventional therapies. This could allow for the suppression of specific inflammatory pathways while leaving the rest of the immune system intact, leading to fewer side effects.
Infectious Diseases
TsAbs can also be engineered to target multiple epitopes on a single pathogen, such as a virus. This approach can prevent viral entry into host cells and reduce the likelihood of the pathogen developing resistance. This multi-targeting capability makes them highly effective in treating complex and rapidly evolving infectious diseases.
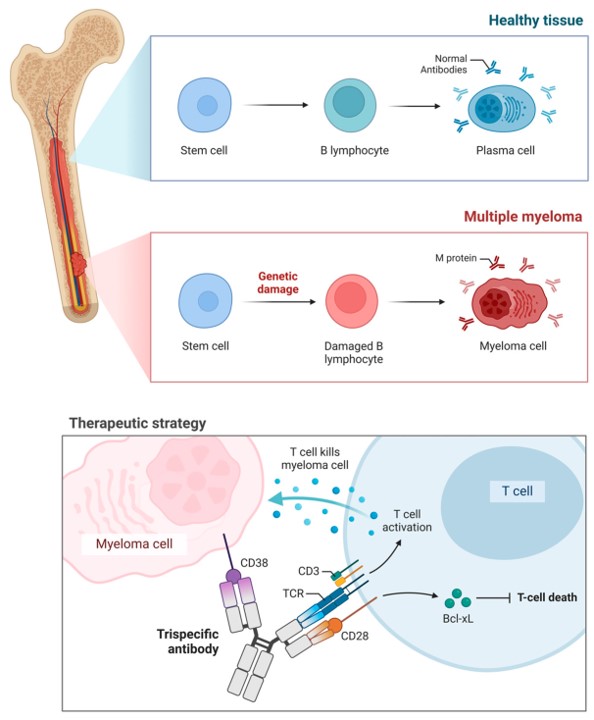 Fig.2 Immunotherapy in multiple myeloma with TsAbs.2,3
Fig.2 Immunotherapy in multiple myeloma with TsAbs.2,3
Service Highlights
-
Advanced Computational Design: We use advanced computational modeling to predict optimal molecular configurations. This ensures each binding arm has high specificity and affinity, minimizing off-target effects and providing a more predictable development path.
-
Proven Manufacturability: Our platform designs molecules that are inherently stable and easy to manufacture. This reduces the risk of aggregation and production issues, ensuring a reliable and scalable supply. Our methods save significant time and resources.
-
Streamlined Development Lifecycle: Our fully integrated service accelerates the entire development process. From initial design to final delivery, our streamlined workflow ensures your project progresses efficiently, allowing you to bring therapies to the clinic faster and more cost-effectively.
Q&A
Q1: What is your process for optimizing the binding affinity of the three arms?
A1: Our process for optimizing binding affinity involves rational design and high-throughput screening. We use advanced computational modeling to predict optimal binding domains and linker lengths. We then perform extensive in vitro screening to select candidates with the desired affinity and specificity. We use our proprietary platform to ensure each arm functions flawlessly without interfering with the others.
Q2: How do you manage the risk of immunogenicity with these complex molecules?
A2: We manage immunogenicity risk by designing our TsAbs with minimized immunogenic epitopes. We use advanced in silico prediction tools to mitigate potential risks early in the design phase. We monitor for immunogenicity throughout the development process with robust analytical assays. We ensure a high level of purity in our final products, a key factor in reducing immunogenicity.
Q3: What is your approach to ensuring a favorable safety profile for the final product?
A3: Our approach to ensuring a favorable safety profile is comprehensive and multifaceted. We perform extensive in vitro and in vivo testing to evaluate potential off-target effects and toxicity. We provide immunogenicity analysis to manage the risk of anti-drug antibody formation. Our designs focus on minimizing the potential for cytokine release syndrome. We ensure all our processes meet the highest standards of quality and compliance.
Q4: Can your services accommodate unique or non-standard trispecific formats?
A4: Our services are designed with a high degree of flexibility to accommodate unique and non-standard trispecific formats, as we are a leading innovator in this field. Our proprietary platform and extensive engineering capabilities allow us to create a wide variety of molecular architectures to meet specific therapeutic needs. We encourage clients to discuss their unique design concepts during the initial consultation.
Q5: Do you offer services for optimizing an existing TsAb lead?
A5: We do offer services for optimizing an existing TsAb lead, as we are committed to helping clients at any stage of their development journey. Our optimization services can focus on improving a range of properties, including binding affinity, half-life, and manufacturability. We can also assist in re-engineering an existing bispecific format into a new trispecific molecule.
The development of TsAbs is a rapidly evolving field with the potential to redefine modern therapeutics. Partner with Creative Biolabs to gain a strategic advantage. Our unparalleled expertise and integrated platform are at your service to transform your therapeutic concepts into a clinical reality. Contact us today to begin your journey.
References
-
Runcie, Karie et al. "Bi-specific and tri-specific antibodies- the next big thing in solid tumor therapeutics." Molecular medicine (Cambridge, Mass.) vol. 24,1 50. 24 Sep. 2018, doi:10.1186/s10020-018-0051-4.
-
Amoozgar, Behzad et al. "From Molecular Precision to Clinical Practice: A Comprehensive Review of Bispecific and Trispecific Antibodies in Hematologic Malignancies." International Journal of Molecular Sciences vol. 26,11 5319. 1 Jun. 2025, doi:10.3390/ijms26115319.
-
Distributed under Open Access license CC BY 4.0, without modification.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY


 Fig.1 Schematic representation of TsAb structure.1,3
Fig.1 Schematic representation of TsAb structure.1,3
 Fig.2 Immunotherapy in multiple myeloma with TsAbs.2,3
Fig.2 Immunotherapy in multiple myeloma with TsAbs.2,3


