Cell level diagnosis plays a significant role in the in vitro diagnostics (IVD) field. Cellular heterogeneity and changes carry the signatures of health and disease; therefore, cell-level diagnosis has been widely utilized for the detection of tumors. Powered by our advanced platforms and experienced experts in IVD development, Creative Biolabs is fully competent to provide high-quality cell-level diagnosis services to facilitate our clients' cancer research project development. We also serve as your reliable partner by tailoring the most appropriate solutions for cell-level diagnosis development.
Cell Level Diagnosis
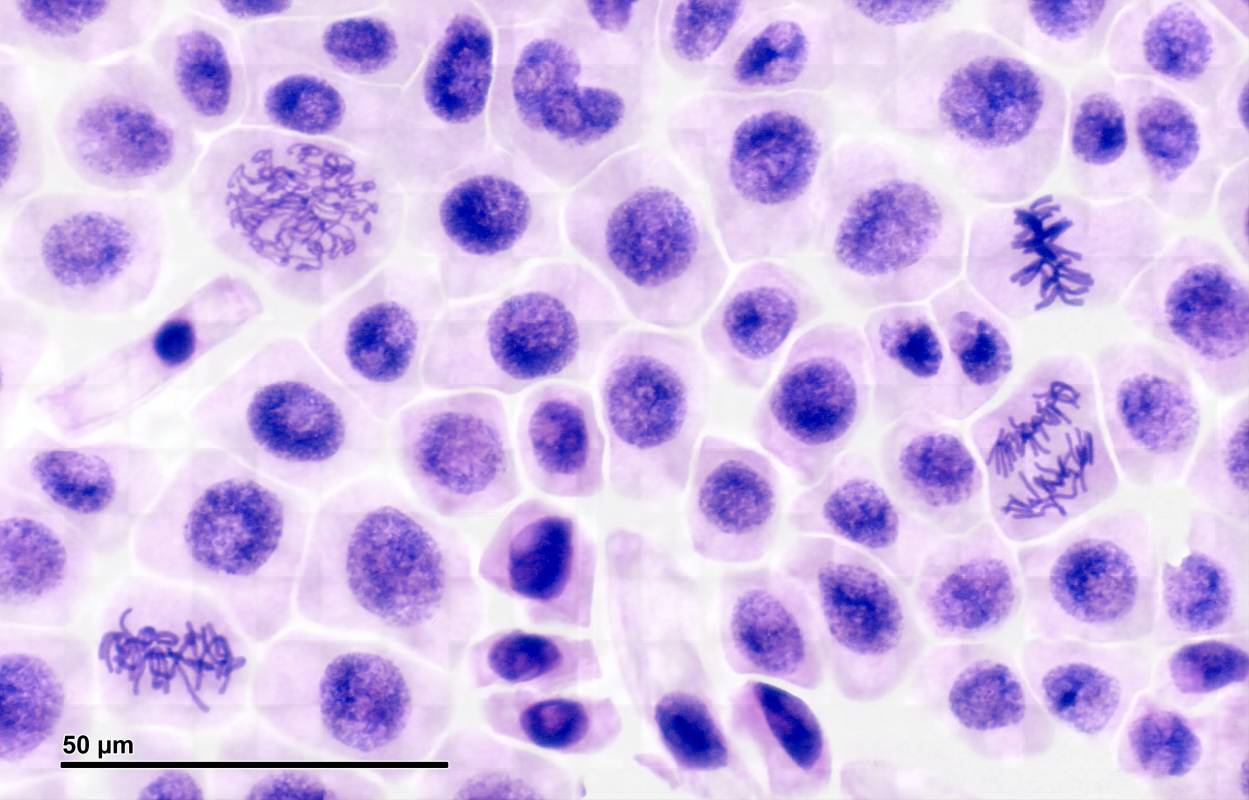
Gene diagnosis, protein diagnosis, tissue diagnosis, and cell diagnosis, are four main categories in the field of IVD, according to the detection level. Cell level diagnosis, such as cell counting, monitoring, or flow cytometry test, can be used to monitor a person's overall health to help cure, treat, or prevent diseases. Especially, cell-level diagnosis may open new frontiers for detection and early prediction of tumors. More work is necessary to fully optimize clinical cell-level diagnosis and to improve the accuracy, specificity and sensitivity. Thinprep Cytologic Test (TCT) and CTC Detection are the most widely used in clinical practice in cell-level diagnosis.
 Distributed under CC BY-SA 3.0, from Wiki, without modification.
Distributed under CC BY-SA 3.0, from Wiki, without modification.
- Thinprep Cytologic Test (TCT)
TCT is a Liquid Based Cytology (LBC) method, which initiates changes in the way of fixation and production of slides which enhance dramatically the smear quality. It has been authorized to serve as a replacement to the conventional Papanicolaou test in 1996 by the Food and Drug Administration (FDA). TCT is intended for use in the screening and detection of cervical cancer, pre-cancerous lesions, atypical cells and all other cytologic categories as defined by the Bethesda system for reporting results of cervical cytology.
Circulating tumor cells (CTCs) are cancer cells that circulate in the bloodstream after being naturally shed from original or metastatic tumors. CTC detection is a liquid biopsy of tumors that can be used for early diagnosis of cancers, earlier evaluation of cancer recurrence, tumor prognosis, monitoring cancer progress and evaluation of chemotherapeutic efficacy, and developing individual sensitive anti-cancer drugs. It is a valuable tool in cell-level diagnosis.
Services at Creative Biolabs
Combined with the expertise and latest cell diagnosis technology, Creative Biolabs provides one-stop cell-level diagnosis services with high precision, sensitivity and flexibility. We have perfected our technical pipelines in the development of cell-level diagnosis for cancer research, especially TCT for cervical cancer and CTCs detection. Our platform facilitates both automated enrichment and flow cytometric analysis of circulating tumor cells, which allows the rapid and reproducible identification, quantification, and characterization of CTCs. Our platform prepares clearer and easier-to-read test slides to increase the racy for assessment of the tumor cells.
Cell-level diagnosis helps inform clinical decision making. If you are interested in our services, please feel free to contact us for more information.
Published Data
1. Development of an Innovative Approach for the Detection of Circulating Tumor Cells
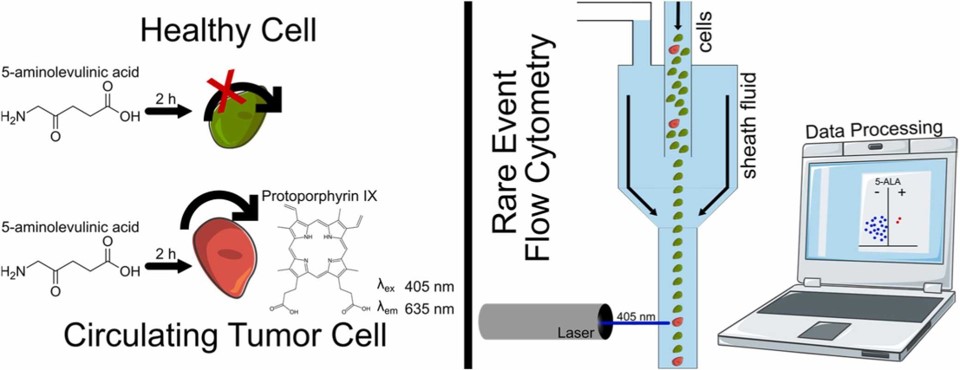 Fig.1 Schematic representation of the CTC detection approach based on flow cytometry and 5-ALA staining.1
Fig.1 Schematic representation of the CTC detection approach based on flow cytometry and 5-ALA staining.1
In this study, researchers developed and evaluated a reliable, affordable CTC screening approach using flow cytometry and 5-aminolevulinic acid (5-ALA)-induced fluorescence staining. They established a circulation model with breast cancer MDA-MB-231 cells as the model cell line, aiming to mimic physiological conditions closely while adhering to the 3R principles, which promote alternatives to animal testing. The method successfully detected an average of 11 ± 3.3 CTCs per 10,000 peripheral blood mononuclear cells, representing approximately 0.1% with a reasonable number of false positive events. In addition, the study explored a theranostic approach using 5-ALA converted to protoporphyrin IX for CTC detection. Results showed that CTCs could be detected after 2 hours of circulation with 2.5 mM 5-ALA exposure. These findings have the potential to advance CTC detection methods and may significantly enhance cancer detection, monitoring, and treatment strategies.
Reference
- Roschenko, Valeri, et al. "An innovative approach to detect circulating tumor cells." Colloids and Surfaces B: Biointerfaces 241 (2024): 114059. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.