Materials and reagents are developed for immunoassays and molecular tests that detect viruses, bacteria, and other pathogens. These components are essential for creating rapid and accurate diagnostic kits for infectious diseases such as influenza and COVID-19.
As a premier provider of in vitro diagnostics (IVD) materials and reagents development services, Creative Biolabs offers end-to-end solutions, from concept to commercialization. Our strengths lie in our technical expertise and rigorous quality systems, providing customers with a seamless pathway to high-performance, market-ready diagnostic products.
IVD Materials and Reagents
IVD is the backbone of modern clinical decision-making, providing essential information about a patient's health, disease state, or response to treatment. These tests are performed outside the body using biological samples such as blood, urine, or tissue. The accuracy and reliability of every IVD assay depend entirely on the quality and performance of its core components: the materials and reagents. These are the fundamental building blocks that enable the detection and measurement of specific biomarkers.
IVD reagents encompass a diverse array of chemical and biological substances, including antibodies, antigens, enzymes, and buffers, which are designed to react with a target analyte. The materials, such as microplates, lateral flow membranes, or consumable plastics, provide the physical platform for these reactions. Ensuring the consistency, stability, and specificity of these components is a critical and often challenging aspect of IVD development, directly impacting the final product's performance and regulatory success.
Our IVD Materials and Reagents Development Services
Our molecular biology and R&D departments have extensive experience in biomarker discovery, cell line development and optimization, peptide synthesis, and recombinant protein expression. We specialize in the development of both monoclonal and polyclonal antibodies. After a robust proof-of-concept phase, our team provides comprehensive IVD materials and reagents development services.
We create custom blocking solutions, enzyme substrates, IVD antibodies, and optimized antibody pairs. To meet diverse project demands, conjugates to haptens, fluorophores, infrared dyes, and chemiluminescent reporters are precisely tailored. We also provide thorough validation and characterization services, including the evaluation of the limit of detection, the limit of blank, limit of quantification, linearity, spiked recoveries, and interfering substances, to ensure the highest quality and regulatory compliance.
Our Services Portfolio Includes:
- IVD Material Development Service
We provide comprehensive development services for critical IVD materials, including custom microplates, lateral flow consumables, and other assay-specific components. Our focus is on manufacturing with precision, bio-inertness, and consistency, creating a reliable platform for diagnostic reactions. This service covers material selection, design, prototyping, and scalable manufacturing, all with stringent quality control for lot-to-lot consistency.
- IVD Reagent Customization Service
Our reagent customization services address the unique needs of your diagnostic platform. We develop and optimize critical reagents like buffers and diluents to enhance assay stability and performance. We also specialize in custom master mixes for molecular diagnostics. Every custom formulation undergoes rigorous stability testing and performance validation to guarantee long-term reliability.
- Glycochemical Product Customization Service
Glycans and glycoconjugates are important in diagnostics as biomarkers for diseases like cancer. Our specialized services focus on the custom synthesis and purification of glycochemical products for IVD assays. We create unique glycan-based antigens and develop reagents to detect specific glycosylation patterns, critical for next-generation diagnostics with enhanced sensitivity.
Service Workflow of IVD Materials and Reagents Development
We begin with a detailed consultation to understand your project scope, technical requirements, and target application.
Our team identifies the critical components and develops a preliminary project plan, assessing the feasibility of the proposed solution.
We create and test preliminary prototypes of the materials and reagents to validate the underlying technology and confirm the concept's viability.
Once the concept is proven, we optimize the product to meet all specifications. This stage includes comprehensive validation to ensure performance and quality.
The final stage involves scaling up manufacturing processes for commercial production and providing the final delivery of your validated, quality-assured IVD materials and reagents.
Applications
Infectious Disease Diagnostics
Oncology and Cancer Biomarkers
Services are provided for developing reagents that detect specific proteins or genetic biomarkers associated with various types of cancer. These materials find use in companion diagnostics, which help guide treatment decisions and monitor disease progression with high precision.
Autoimmune and Hormonal Assays
Custom reagents are supplied for immunoassays that measure hormone levels and detect autoantibodies. These components are vital for developing diagnostic tests for autoimmune diseases, including thyroid disorders, diabetes, and rheumatoid arthritis.
Personalized Medicine and Genetic Testing
Molecular reagents, including custom primers and probes, are utilized in the development of genetic tests. These materials are fundamental for creating assays that identify genetic variations, predict drug response, and enable a personalized approach to patient treatment.
Published Data
1. Development of a Fluorescent Lateral Flow Immunoassay Utilizing Aggregation-Induced Emission Carbon Dots
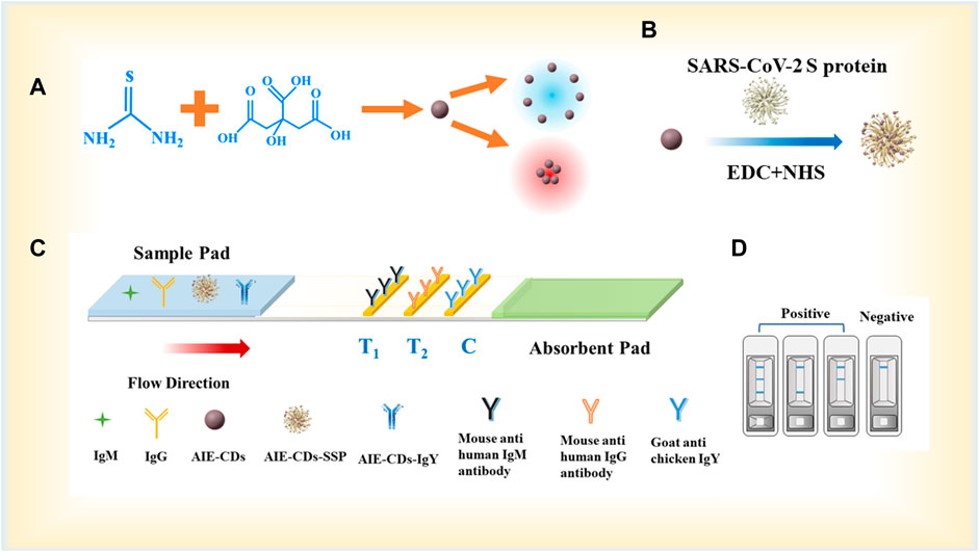 Fig.1 Fabrication of the LFA strip.1,3
Fig.1 Fabrication of the LFA strip.1,3
In this study, researchers first introduced aggregation-induced emission carbon dots (AIE-CDs) with dual-emission peaks as a novel fluorescent material for immunoassays, and the AIE-CDs were used to develop a lateral flow immunoassay (LFA) for detecting SARS-CoV-2-specific IgM and IgG antibodies. AIE-CDs are known for their strong emission efficiency even in aggregated or solid-state forms. In this work, the AIE-CDs were synthesized and conjugated to the SARS-CoV-2 spike protein (SSP) using the carbodiimide method, forming a fluorescent complex (AIE-CD-SSP). The assay utilized a sandwich structure (antigen–antibody–anti-antibody) to specifically detect the antibodies. The fluorescent immunochromatographic test strips enabled the simultaneous and separate detection of IgM and IgG with a detection limit as low as 100 pg/mL. This innovative approach offers a highly sensitive and efficient diagnostic tool, with potential applications in public health and clinical research for SARS-CoV-2 detection.
Service Highlights
- End-to-End Solutions: Our comprehensive services, from biomarker discovery to final production, provide a seamless and efficient development process that minimizes risks and accelerates your time to market.
- Technical and Regulatory Expertise: With deep technical knowledge and a strong understanding of regulatory requirements, our team ensures your products are both high-performing and compliant with stringent industry standards from the outset.
- Scalable Manufacturing Capabilities: We offer flexible, scalable manufacturing options to support your project at any stage, from small-scale validation to high-volume commercial launch, all without compromising quality.
- Assured Quality and Consistency: Every material and reagent we develop undergoes rigorous quality control testing to ensure lot-to-lot consistency. Our robust quality management system guarantees that all products deliver dependable and reproducible results.
FAQs
-
Q: How do you ensure the quality and consistency of the IVD materials and reagents you develop?
A: Our team employs a comprehensive quality management system that governs every stage of the development and manufacturing process. We conduct thorough raw material qualification, in-process quality control checks, and rigorous final product testing to ensure lot-to-lot consistency.
-
Q: Do you offer support for the scale-up and high-volume manufacturing of IVD reagents?
A: Our company is equipped with scalable manufacturing capabilities designed to support projects from the initial validation stage to commercial production. We collaborate with you to ensure a smooth transition from R&D to large-scale manufacturing without compromising quality.
-
Q: What kind of support do you provide after the delivery of the final product?
A: We offer ongoing technical support and customer service after the final product has been delivered to ensure its continued performance. We are available to assist with any questions or issues that may arise, providing continuous partnership and support for your diagnostic products.
-
Q: How do you address the technical challenge of interfering substances in complex biological samples?
A: We have a dedicated team of experts who specialize in developing advanced blocking reagents and formulation strategies. We work to mitigate potential interferences from complex biological matrices, such as blood or serum, to enhance the specificity and reliability of your assay.
-
Q: What types of conjugation services do you offer for antibodies and other reagents?
A: We offer a wide range of conjugation services, including the precise tailoring of haptens, fluorophores, infrared dyes, and chemiluminescent reporters. Our expertise ensures that your conjugates are optimized for performance in various diagnostic platforms.
-
Q: Is it possible to get a custom IVD antibody pair developed for my specific sandwich immunoassay?
A: We have a dedicated service for identifying and developing custom antibody pairs, which is essential for creating high-performance sandwich immunoassays. Our team works to optimize the pair for maximum sensitivity and specificity for your target analyte.
Creative Biolabs can provide a global solution for academic diagnostic research, with targeted support and a focus on customer satisfaction. Questions about immunoassay materials? We're happy to answer any questions you have regarding our products and services! Contact us for more information.
References
- Ju, Jian, et al. "Development of fluorescent lateral flow immunoassay for SARS-CoV-2-specific IgM and IgG based on aggregation-induced emission carbon dots." Frontiers in Bioengineering and Biotechnology 10 (2022): 1042926.
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.