Custom-formulated antibodies are essential for therapeutic applications like treating cancer and autoimmune disorders. A stable formulation ensures consistent dosing and efficacy while mitigating the risk of immunogenicity by reducing aggregate formation. This contributes directly to patient safety and the overall success of a therapeutic drug.
Monoclonal antibodies (mAbs) are central to biologics development. Creative Biolabs' advanced in vitro diagnostics (IVD) antibody platform offers comprehensive custom services for monoclonal and polyclonal antibody generation. We provide precise solutions for diverse antibody formulations. Our experienced chemists develop novel and optimized antibody products, creating high-quality, custom reagents tailored to client specifications for competitive, high-performing antibodies.
Antibody Formulation
Antibody formulation is a critical discipline in biopharmaceutical development, aimed at preserving the structural integrity and biological activity of antibodies throughout their lifecycle. Antibodies are inherently susceptible to various degradation pathways, including aggregation, fragmentation, oxidation, and deamidation, which can compromise efficacy and increase immunogenicity. These degradation events are influenced by intrinsic factors such as amino acid sequence, pI, and glycosylation, as well as external stressors like temperature, pH, and mechanical agitation. Therefore, strategic formulation, involving the precise selection of excipients and control of environmental conditions, is essential to confer long-term stability and ensure optimal therapeutic performance.
Our Custom Formulation Development Services
Most antibodies are stored in buffers containing preservatives, stabilizers, or other excipients, such as sodium azide, bovine serum albumin (BSA), and glycerol. Our scientists specialize in custom formulation development for various antibody types, including IgG, single-chain variable fragments (scFv), antigen-binding fragments (Fab), bispecific antibodies (BsAbs), and single-domain antibodies (sdAbs). Following an initial consultation, our team initiates antibody formulation at small scales, producing several trial batches for evaluation. After rigorous testing of these samples, a finalized formulation is established. Our technology will meet clients' needs from early-stage research through to commercial production.
Service Workflow of Custom Formulation Development
Our project workflow is a systematic and collaborative process, meticulously designed to ensure optimal results for your antibody. The journey is broken down into the following key steps:
We begin with a detailed discussion to fully understand your antibody's properties, intended application, and stability requirements. This establishes the foundation and specific goals for the entire project.
Our team conducts a comprehensive biophysical analysis of the antibody to identify its unique physicochemical properties and inherent degradation pathways, such as aggregation and oxidation.
We systematically screen and test various excipients to identify the most suitable combination of stabilizers, buffers, and surfactants that work synergistically with your antibody.
Small-scale trial batches are developed and prepared using the selected excipients, often employing high-throughput methods for efficiency.
These formulations undergo rigorous, forced degradation studies under various stress conditions, including thermal and freeze-thaw cycles, to assess their robustness.
Based on the stability data, we iteratively optimize the components and finalize the most effective and robust formulation for your specific needs.
The project concludes with the delivery of the finalized formulated product along with a detailed and comprehensive report of the entire development process.
Applications
Therapeutic Development
In Vitro Diagnostics
Formulated antibodies are used in diagnostic assays such as ELISA and Western blotting. Their stability is crucial for generating reliable and reproducible results in a clinical setting. A robust formulation prevents lot-to-lot variability and ensures consistent binding affinity, which is essential for accurate disease diagnosis.
Research and Drug Discovery
High-quality, stable antibodies are critical tools for academic and industrial research. A consistent formulation prevents experimental artifacts and batch-to-batch variation, ensuring data integrity. This reliability is fundamental for advancing scientific knowledge and for identifying new therapeutic candidates.
Bioanalytical Testing
These formulated antibodies serve as key reagents in bioanalytical methods used to quantify drug concentrations in biological samples for pharmacokinetic and pharmacodynamic studies. A properly formulated antibody is essential for developing a sensitive and specific assay, a crucial step for clinical trials and regulatory submissions where precise data is required.
Published Data
1. Development of Trehalose-Based Excipients for Enhancing Anti-SARS-CoV-2 Antibody Stability
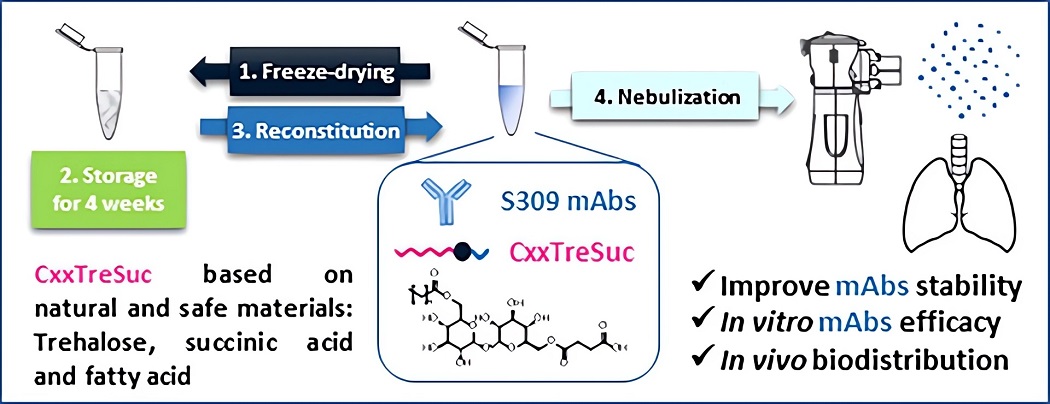 Fig.2 Generation and validation of trehalose-based excipients.1,3
Fig.2 Generation and validation of trehalose-based excipients.1,3
In this work, researchers synthesized a novel series of excipients consisting of a trehalose polar head, a succinyl moiety, and different hydrophobic carbon chains ranging from 8 to 16 carbon. Succinylation improved the solubility of these excipients, enabling their use at effective concentrations for protein stabilization. Notably, the excipient with a 16-carbon chain (C16TreSuc), when used at 5.6 mM, successfully preserved the colloidal stability and antigen-binding activity of an antibody during nebulization. It also acted as a cryoprotectant, enabling the long-term storage of antibodies in lyophilized form for weeks. The study demonstrated that C16TreSuc could stabilize antibodies for COVID-19 treatment. In vitro, it preserved and even enhanced the neutralization capacity of an antibody against SARS-CoV-2 during nebulization. In vivo, nebulization with C16TreSuc resulted in widespread distribution of the anti-SARS-CoV-2 antibody in mice lungs. This work explores trehalose-based excipients as potential replacements for traditional stabilizers like polysorbates in protein lung delivery.
2. Development of Monoclonal Antibody Formulation via Combining HPβCD and Polysorbate
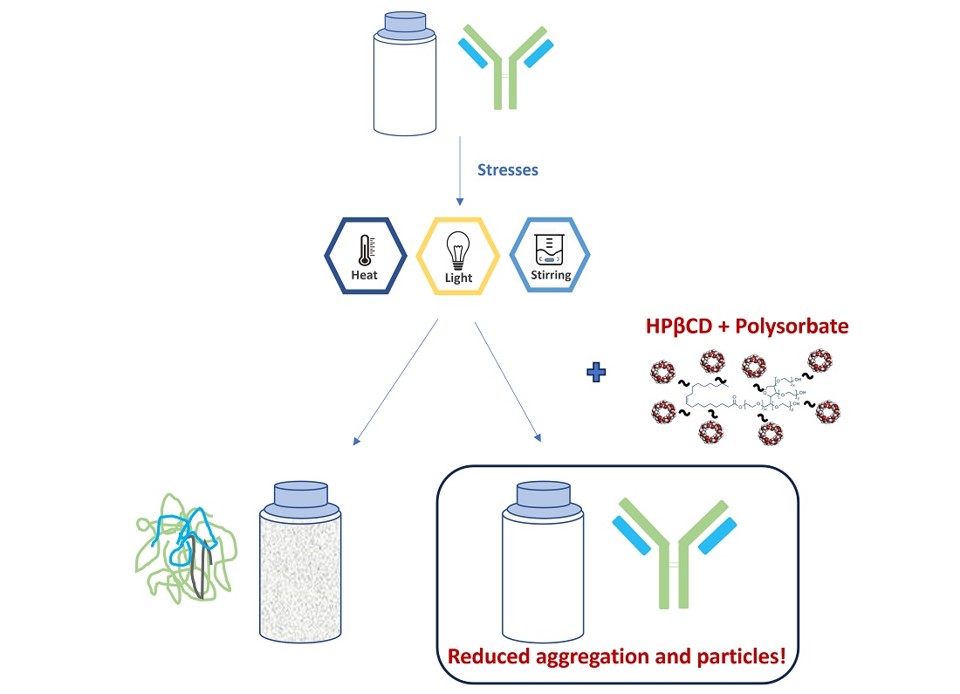 Fig.3 Stabilizing effects of HPβCD and polysorbate combination on mAbs.2,3
Fig.3 Stabilizing effects of HPβCD and polysorbate combination on mAbs.2,3
This study explored the combined effects of hydroxypropyl beta-cyclodextrin (HPβCD) and polysorbates as excipients in protein formulations. HPβCD significantly reduced aggregation in two mAbs models under various stress conditions. The diffusion interaction parameter indicated that HPβCD lowered protein-protein interactions. Notably, the combination of HPβCD and polysorbates not only decreased antibody aggregation but also reduced subvisible particle counts after agitation, light exposure, and heat stress, outperforming commercial formulations. ITC and surface activity measurements suggested that HPβCD enhanced the surface activity of polysorbates, contributing to improved stability. The findings highlight the potential of this combination to enhance mAb stability, offering an alternative stabilizer strategy for the biopharmaceutical industry.
Service Highlights
- Specialized Expertise: Our team of formulation chemists possesses deep expertise in the unique challenges of antibody stability and degradation, providing a highly specialized service for biologics.
- Data-Driven Approach: We employ a robust, data-driven methodology, including comprehensive stability studies and excipient screening, to create formulations with a scientifically proven track record of success.
- Seamless Project Management: Our streamlined workflow ensures clear communication and a collaborative partnership from initial consultation through to the final delivery of the formulated product.
- Flexible Solutions: We offer tailored solutions for a wide range of antibody formats, from traditional IgGs to single-domain antibodies, addressing the diverse needs of our clients.
FAQs
-
Q: What is the primary benefit of a custom formulation for my antibody?
A: The primary benefit of a custom formulation is the enhanced stability and extended shelf life of your antibody. A tailored formulation minimizes degradation pathways, such as aggregation and oxidation, which are common with biologics. This specialized approach ensures that the antibody retains its full activity and integrity for longer periods, providing a more reliable and consistent product for your specific application.
-
Q: What types of excipients do you typically use in your formulations?
A: We select excipients based on the unique properties of your specific antibody to ensure optimal stability. Common classes of excipients we utilize include surfactants like polysorbates to prevent aggregation, stabilizers such as trehalose or sucrose for cryoprotection, and various buffers to maintain a stable pH. The final choice of excipients is determined by a systematic screening process to identify the most effective combination.
-
Q: How do you address the issue of aggregation in antibody formulations?
A: We address the critical issue of antibody aggregation through a multi-faceted approach, beginning with a thorough biophysical characterization of the molecule. Our process involves screening a variety of excipients, particularly surfactants and osmolytes, that are known to minimize protein-protein interactions. We then test these candidate formulations under various stress conditions to identify the most effective formulation for reducing aggregation and preserving the antibody's colloidal stability.
-
Q: Can you develop a formulation for a specific delivery method, such as nebulization?
A: Yes, we are fully capable of developing a formulation optimized for a specific delivery method. Our scientists have experience with a range of delivery systems, including nebulized and lyophilized products. We will design a formulation that not only stabilizes the antibody but also ensures it remains functional and effective throughout the specific manufacturing process and mode of administration.
-
Q: How does your process ensure the final formulation is safe and non-immunogenic?
A: Our process is meticulously designed to ensure the final formulation is both safe and non-immunogenic. We exclusively use excipients that are widely recognized as safe for pharmaceutical use and are carefully tested for compatibility. Furthermore, by preventing antibody degradation and aggregation, our formulations minimize the formation of immunogenic degradation products, thus reducing the potential for an adverse immune response.
-
Q: What kind of stability testing do you perform on the trial batches?
A: We perform a comprehensive suite of stability tests on all trial batches to evaluate the formulation's performance. These tests include accelerated stability studies under thermal stress and freeze-thaw cycles, as well as real-time stability assessments. We use analytical techniques such as size exclusion chromatography (SEC) to monitor aggregation and particle analysis to measure colloidal stability.
Creative Biolabs has the necessary expertise and capabilities to evaluate each antibody, provide high-quality custom formulation development service and ensure we are preparing the most suitable formulation. If you are seeking an antibody in a different formulation, our seasoned scientists can help. For more detailed information, please feel free to contact us or directly send us an inquiry.
References
- Noverraz, François, et al. "Novel trehalose-based excipients for stabilizing nebulized anti-SARS-CoV-2 antibody." International Journal of Pharmaceutics 630 (2023): 122463.
- Huang, Jiayi, et al. "Investigation on the Combined Effect of Hydroxypropyl Beta-Cyclodextrin (HPβCD) and Polysorbate in Monoclonal Antibody Formulation." Pharmaceuticals 17.4 (2024): 528.
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.