With the unique features of miniaturization, high integration, and low cost, Microfluidics is emerging as a powerful tool for disease diagnosis and monitor. Creative Biolabs offers unique and adaptable Microfluidic-based detection and analysis services for in vitro diagnostics (IVD) of different diseases. Armed with the advancement of the cloud, digital medicine, and nanotechnology, the integration of new emerging elements would bring advances to assay sensitivity, stability, and robustness.
Technology Introduction
Microfluidics, or lab-on-a-chip technology, is a powerful tool that sits at the intersection of biotechnology, automation, and functional integration. By using different fabrication techniques to make fluid flow cells, Microfluidic devices can shuttle picoliter, volumes of fluids into functional regions, enabling powerful IVD harnessing everything from digital biology, next generation sequencing, organs-on-a-chip, high-throughput cell-based assays, or high-density multifunctional chips for drug discovery and bioanalysis for shrinking sample and reagent volumes.
Application of Microfluidics in IVD
Early detection of diseases such as HIV/AIDS, cancer, and chronic respiratory diseases has the potential to reduce mortality rates, and portable, easy-to-use, and affordable diagnostic methods/products provide an optimal platform for disease recognition. The majority of Microfluidic products that have been recently developed work by sensing specific biomarkers, human cell types, bacteria, or viruses. Four high-impact applications for Microfluidic diagnostic research include infectious diseases, autoimmune diseases, cancer, and cardiovascular diseases.
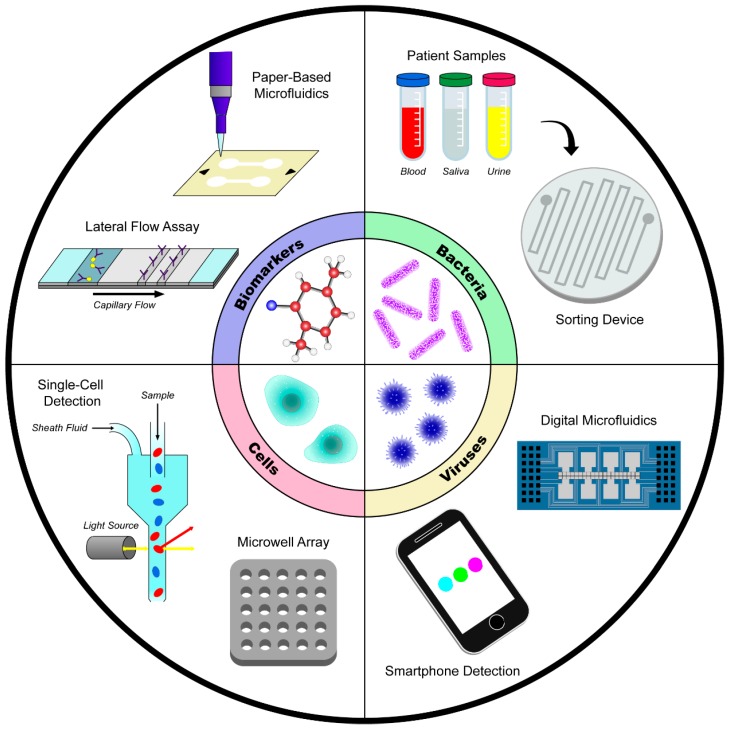 Fig.1 Microfluidic technology for biomarker, human cell, bacteria, and virus detection.1, 2
Fig.1 Microfluidic technology for biomarker, human cell, bacteria, and virus detection.1, 2
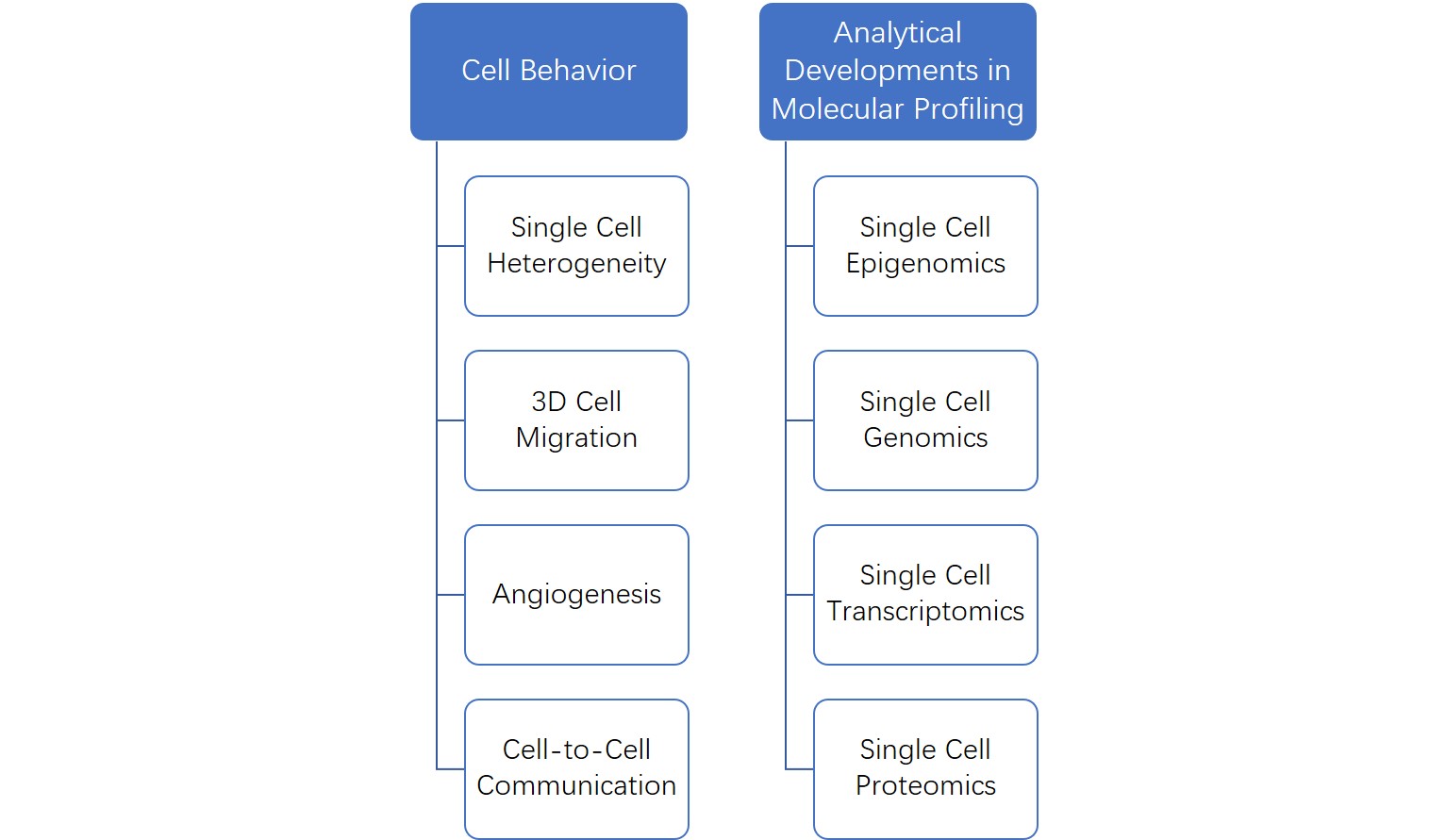
Microfluidic approaches have also been well suited to investigate single cell behavior due to their increased sensitivity, economy of scale and ease of automation. This technique plays a critical role in delineating heterogenetic cellular sub-populations by providing phenotypic-genotypic correlations, eventually contributing to an effective understanding of personalized nature of the disease.
Incomparable Advantages Over Conventional Detection Techniques
- Microfluidics allows for highly sensitive and controllable conditions by facilitating the predictable passage of fluids through microchannels due to laminar flow.
- Manipulated sensitivity to provide researchers with ways to produce detection and single cell analysis methods that cannot be recreated on a larger scale.
- Microfluidics provides integrated approaches for the individual analyte or multiplexed protein and nucleic acid analysis.
Our Featured Services
At Creative Biolabs, Microfluidic technology makes full use of advantages in the field of disease detection and their use in diagnostic understanding. Creative Biolabs now provides the following services based on Microfluidic technology. Please refer to corresponding page for details.
 Internet of Things based Microfluidic System Development Services
Internet of Things based Microfluidic System Development Services
 Microfluidic-based POCT Development Services
Microfluidic-based POCT Development Services
If you are interested in our services, please feel free to contact us for more information.
Published Data
1. Integrated Microfluidics with Optical Label-Free Biosensor for Blood Biomarker Detection
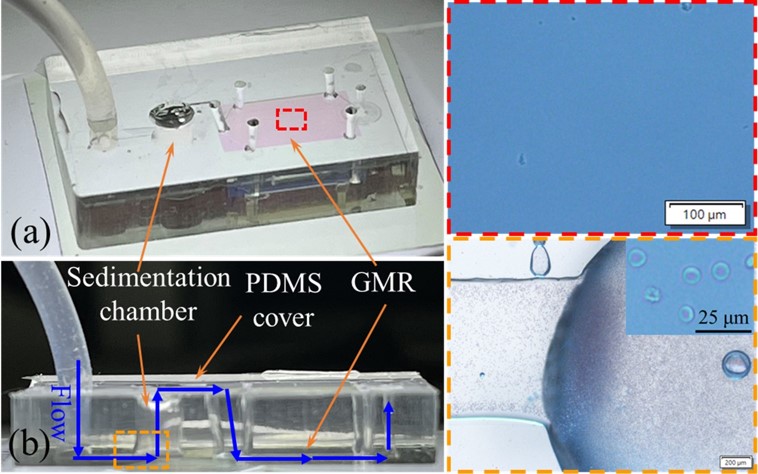 Fig.2 Overview of the optofluidic chip.3,2
Fig.2 Overview of the optofluidic chip.3,2
In this study, researchers presented an integrated optofluidic chip by incorporating a guided-mode resonance (GMR) sensor into a microfluidic system for simultaneous separation of blood plasma and label-free detection of albumin. The microfluidic chip included a sedimentation chamber that separated plasma from blood cells based on density differences. Blood samples were introduced in two phases with regulated flow rates, trapping the cells in the sedimentation chamber and allowing only the plasma to flow toward the GMR sensor for albumin detection. The GMR sensor, produced through plastic replica molding, demonstrated a bulk sensitivity of 175.66 nm/RIU and a detection limit of 0.16 μg/mL for recombinant albumin. This chip shows potential for clinical biomarker detection in point-of-care settings.
2. Development of Compartmentalized Microfluidics for Studying Immune Cells Cross-Talk
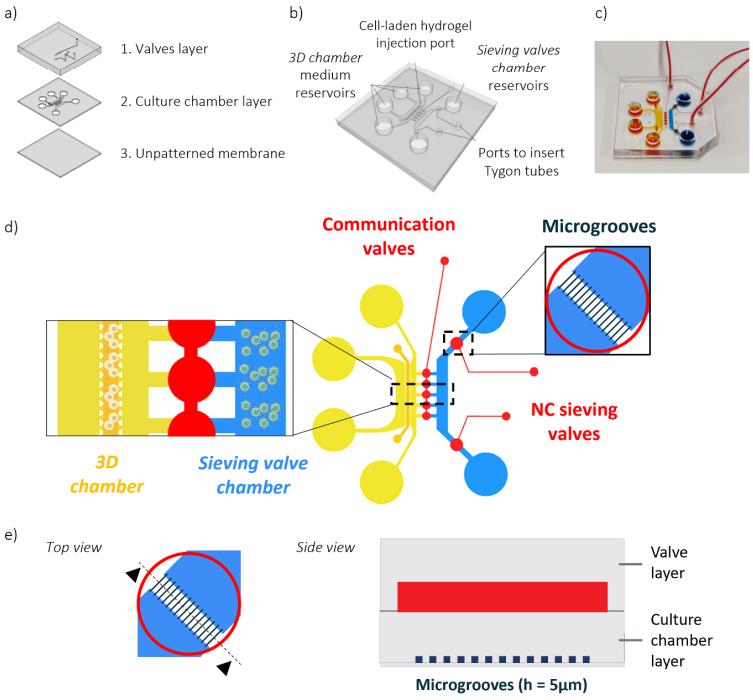 Fig.3 Device concept of the compartmentalized microfluidics.4,2
Fig.3 Device concept of the compartmentalized microfluidics.4,2
This study introduced a compartmentalized microfluidic platform engineered to accurately confine circulating immune cells in organs-on-chip models. The platform integrated innovative normally-closed sieving valves, enabling minimal waste of biological material while co-culturing different immune cell types, such as macrophages and Th1 cells. It allowed separate stimulation of cell subsets and assessment of their cross-talk at specific time points. Functional validation demonstrated the platform's ability to create stable chemotactic gradients for evaluating Th1 cell migration. A proof-of-concept study replicated immune interactions seen in rheumatoid arthritis by assessing Th1 T cell migration towards pro-inflammatory macrophages. These results highlight the platform’s potential for studying immune cell cross-talk and migration in various immune-mediated diseases.
References
- Campbell, Joshua M., et al. "Microfluidic and paper-based devices for disease detection and diagnostic research." International journal of molecular sciences 19.9 (2018): 2731.
- Distributed under Open Access license CC BY 4.0, without modification.
- Li, Chiung-Hsi, et al. "Blood Biomarker Detection Using Integrated Microfluidics with Optical Label-Free Biosensor." Sensors 24.20 (2024): 6756.
- Palma, Cecilia, et al. "A compartmentalized microfluidic platform to investigate immune cells cross-talk in rheumatoid arthritis." Biofabrication 17.1 (2024): 015008.
For Research Use Only.