Hitting roadblocks getting your IVD assays to pass muster on accuracy, reliability, and turnaround time? Our next-gen enzyme-linked immunosorbent assay (ELISA) tech supercharges diagnostic development - get compliant assays to market sooner with smarter processes and direct access to our assay specialists who've been in your lab shoes.
Overview of ELISA
ELISA's become the go-to workhorse in today's biochem labs - delivering the perfect trio: pinpoint accuracy, bulletproof reliability, and workflows your newbies can run. For teams cracking medical mysteries, this isn't just another lab technique, it's engine revving up tomorrow's diagnostic gold standards and treatment game-changers. ELISA encompasses several distinct formats. Below is a detailed exploration of these ELISA types.
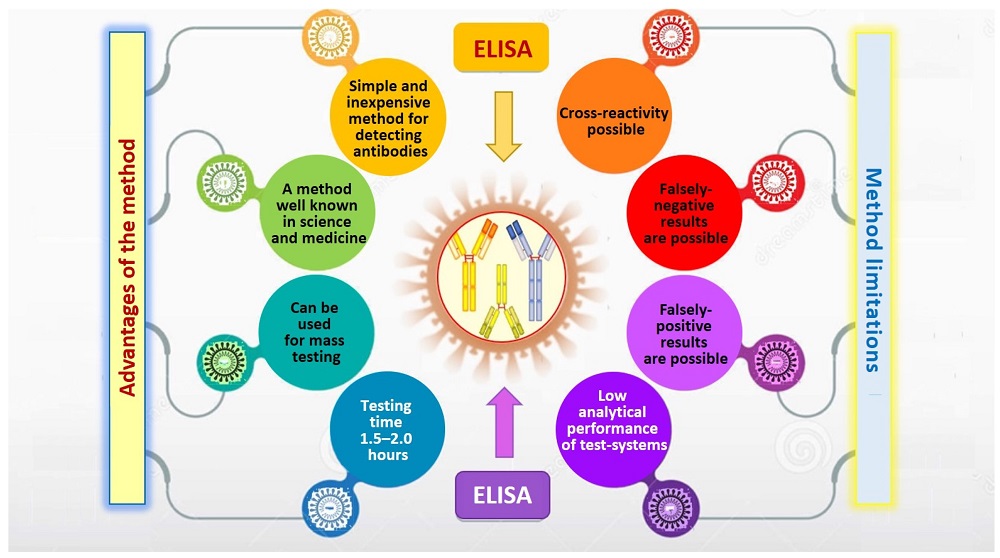 Fig.1 Advantages and limitations of the enzyme-linked immunosorbent assay (ELISA).1
Fig.1 Advantages and limitations of the enzyme-linked immunosorbent assay (ELISA).1
1. Direct ELISA
- Principle: Antigens adhere directly to microplates. An enzyme-linked detection antibody binds and triggers a quantifiable signal (e.g., color change). Ideal for rapid screening.
-
Advantages:
- Simplicity: Direct ELISA involves a single antibody-antigen interaction step, streamlining the assay process.
- Speed: With fewer steps, this format enables rapid results, making it ideal for high-throughput screening.
2. Indirect ELISA
- Principle: Antigens are immobilized on the plate, followed by an unlabeled primary antibody. A labeled secondary antibody then amplifies the signal, boosting sensitivity. Perfect for serological testing.
-
Advantages:
- Flexibility: A vast array of labeled secondary antibodies is commercially available, enabling the use of a single labeled antibody for multiple primary antibodies.
- Signal Amplification: The secondary antibody can bind multiple primary antibodies, enhancing the signal and thereby increasing the assay's sensitivity compared to Direct ELISA.
3. Sandwich ELISA
- Principle: Capture antibody immobilizes antigens from complex samples. A second, enzyme-linked antibody binds a distinct epitope, forming a sandwich. This dual recognition minimizes cross-reactivity, making it ideal for biomarker research.
-
Advantages:
- High Specificity and Sensitivity: The antigen is selectively captured and detected, minimizing non-specific binding and enhancing the assay's accuracy.
- Quantitative Capability: Sandwich ELISA is well-suited for quantifying antigens in complex samples, such as serum, plasma, or cell culture supernatants.
4. Competitive ELISA
- Principle: Sample antigens compete with labeled antigens for limited binding sites. The more unlabeled antigens present, the fewer labeled molecules bind, creating an inverse signal relationship. Excels in quantifying small molecules in complex matrices.
-
Advantages:
- Versatility: Competitive ELISA is particularly useful for detecting small antigens or antigens with a single available epitope, where other ELISA formats may be unsuitable.
- Quantitative Analysis: By comparing the signal intensity to a standard curve generated using known antigen concentrations, the concentration of the antigen in the sample can be accurately determined.
What Can We Do?
Creative Biolabs offers comprehensive ELISA solutions tailored to your IVD development needs. We deliver reproducible and scalable assays, enabling you to bring your diagnostic products to market faster. Our services encompass the entire assay development lifecycle, from initial feasibility studies to final validation.
Key Steps
Details: We evaluate assay requirements, target traits, and resources, considering use (qualitative/quantitative), throughput, and instrumentation. Preliminary experiments test formats, reagents, and conditions; challenges like matrix effects are identified. Outcome: Detailed protocol and feasibility report with risk assessment.
Details: For antibody-based assays, we pick high-affinity, specific antibodies and optimize key reagent concentrations. For antigen-based ones, we use high-purity antigens and refine incubation parameters. Outcome: Optimized reagent sets, conditions, and specs.
Details: We determine LOD, LOQ, linearity, accuracy, precision (intra/inter-assay), and specificity. Robustness is tested under varied conditions, following FDA, EMA, and ICH guidelines. Outcome: Validated assay protocol and comprehensive report.
Details: We handle the transfer, provide training, and detailed protocols/SOPs. For high throughput, we optimize for automation and boost capacity. Outcome: Successful transfer/scale-up with full documentation and support.
Details: The report includes assay description, validation results, and SOPs, presented clearly with stats and visuals. It's reviewed for accuracy. Outcome: Detailed final report with all supporting documents.
Final Deliverables
- A fully validated ELISA assay protocol, including detailed step-by-step instructions, reagent specifications, and quality control procedures.
- Comprehensive data analysis reports, including all raw data, processed data, statistical analyses, and graphical representations of the validation data.
- Detailed standard operating procedures (SOPs) for the assay, covering all aspects of assay performance, including reagent preparation, sample handling, assay procedure, data analysis, and quality control.
Estimated Timeframe
The typical turnaround for ELISA development spans 6–16 weeks, with the exact timeline hinging on multiple interconnected factors, including assay technical complexity, reagent availability, and project-specific parameters. Key determinants include:
- Number of target biomarkers under investigation
- Required sensitivity/specificity thresholds
- Sample matrix complexity (e.g., serum vs. tissue extracts)
- Regulatory compliance requirements
We establish a transparent project timeline during the initial planning phase, complete with milestone checkpoints. Throughout the development process, you'll receive regular progress updates detailing:
- Assay optimization milestones
- Validation data packages
- Any contingency planning for potential roadblocks
This structured approach ensures both technical rigor and client alignment at every stage of development.
Case Study
The client provides a protein target X, which is highly homologous in humans and mice. The project is divided into the following stages, namely peptide immunogen design, peptide preparation and carrier conjugation, animal immunization with KLH-peptide, then cell fusion, hybridoma screening by biotin-peptide coated ELISA, supernatant test, clone subcloning and stabilizing and optional mAb production.
Typically, five mice were immunized. Following the third and fourth injections, serum samples were collected, and the antiserum titer against the target protein was determined by indirect ELISA. The mouse exhibiting the highest titer (Mouse 1) was selected for cell fusion.
A substantial number of positive clones were generated for this project, with approximately 500 clones initially identified and screened by ELISA. Following the first round of specific ELISA screening, nearly 100 clones displayed strong positive signals against the biotin-peptide. After a secondary specific ELISA screening, 30 clones were ultimately selected. Subsequently, hybridoma subcloning was performed twice. Finally, 25 positive strains were identified.
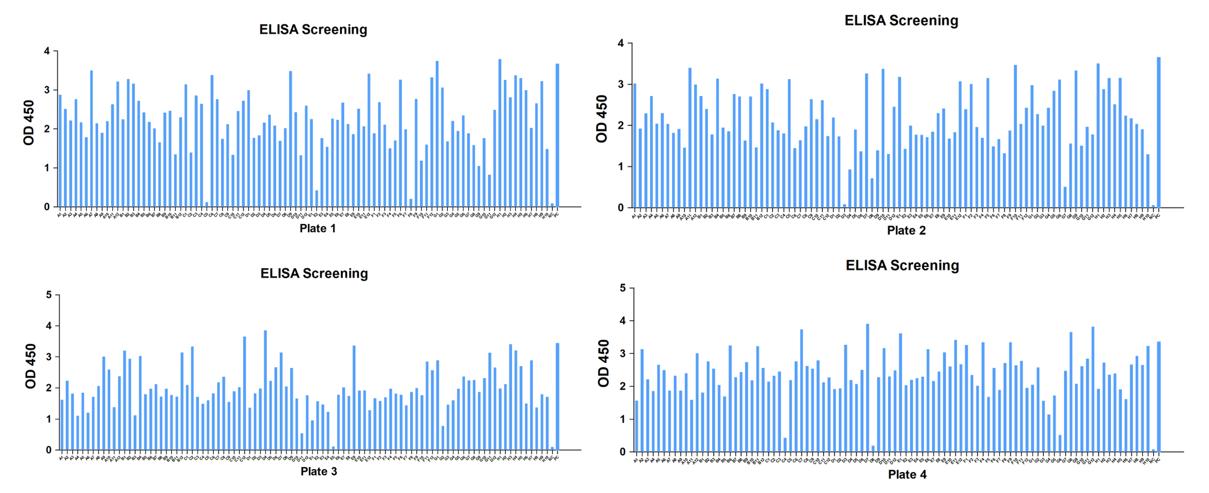 Fig.3 First round specific ELISA screening of clones. |
|
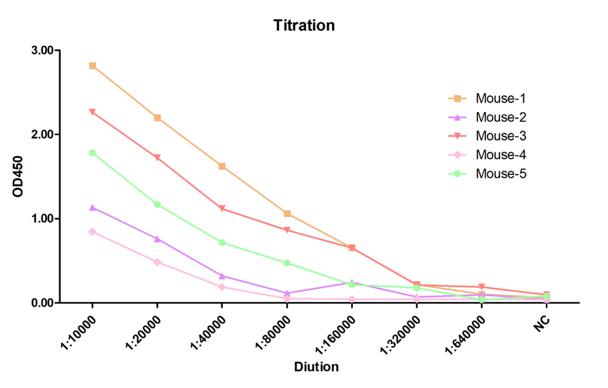 Fig.4 2nd titration result. |
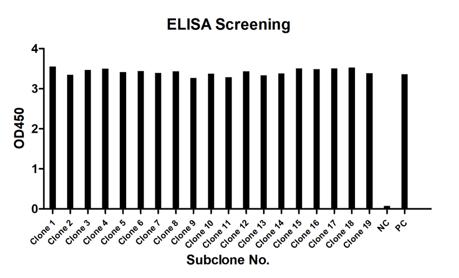 Fig.5 ELISA screening of 2nd subclone. |
Why Choose Us?
- Expertise Rooted in Science
Our team thrives on ELISA and IVD innovation, backed by advanced degrees in immunology, biochemistry, and molecular biology. Decades of hands-on assay design, optimization, and troubleshooting ensure we adapt swiftly to scientific and regulatory shifts.
- Tailored Solutions
No two IVD projects are alike. We partner directly with you to refine assay designs, source specialty reagents, or redesign validation strategies—solving complex challenges with precision and creativity.
- Uncompromising Validation
Rigorous, standards-driven validation (FDA, EMA, ICH) is non-negotiable. We leverage only vetted tools and protocols, delivering bulletproof data that meets global compliance requirements.
- Speed Without Shortcuts
Time-sensitive? Our optimized workflows and transparent communication accelerate timelines while upholding quality. Track progress in real-time—no surprises, no delays.
- Comprehensive Support
Support doesn't end at handoff. From initial setup to long-term data analysis, we're your dedicated scientific ally, committed to your sustained success.
FAQs
Q: How do I optimize ELISA sensitivity?
A:
- Antibody Pairing: Use validated, high-affinity antibodies (e.g., monoclonal pairs for sandwich ELISA).
- Sample Prep: Avoid high-fat/lipid samples (centrifuge to clarify).
- Detection: Switch to fluorescent/luminescent substrates (e.g., HRP + TMB for colorimetric, or chemiluminescent substrates for ultra-sensitivity).
Q: Why am I getting false positives/high background?
A: Common culprits:
- Incomplete Washing: Residual antibodies cause noise.
- Contamination: Use fresh tips, and avoid cross-contamination between standards/samples.
- Over-incubation: Stop the reaction promptly when the color develops.
Related Services
Creative Biolabs offers a comprehensive suite of services to support your IVD development efforts. In addition to ELISA development, we provide:
- Monoclonal Antibody Development
- Polyclonal Antibody Development
- Recombinant Antibody Development
- Antibody Pairs Development
For tailored insights into our ELISA services and to dive into how we can align our expertise with your project's unique demands, don't hesitate to reach out.
Reference
- Andryukov, Boris G., et al. "Laboratory-based resources for COVID-19 diagnostics: Traditional tools and novel technologies. A perspective of personalized medicine." Journal of Personalized Medicine 11.1 (2021): 42. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.