Creative Biolabs is an outstanding expert in the development of in vitro diagnostics (IVD) products based on our excellent scientific team and powerful technology platforms. We support a sensitive and flexible IVD assay development service including fluorescent microsphere-based lateral immunochromatographic assay.
Introduction of Fluorescent Microsphere-based Lateral Immunochromatographic Assay
Lateral immunochromatographic assay is designed to determine the presence or absence of target analyte. The assay is based on the specific binding of antibodies and antigens and label technique, as same as the ELISA sandwich technique. But unlike ELISA, this method is performed on chromatographic paper by capillary action. Fluorescent microsphere-based lateral immunochromatographic assay is an innovative lateral immunochromatographic assay that uses fluorescent microspheres as a labeling probe. Due to several advantages such as simplicity, speediness, and sensitiveness, fluorescent microsphere-based lateral immunochromatographic assay has been used as a promising diagnostic tool. Compared to colloidal gold-based immunochromatographic assay, this assay has a higher sensitiveness.
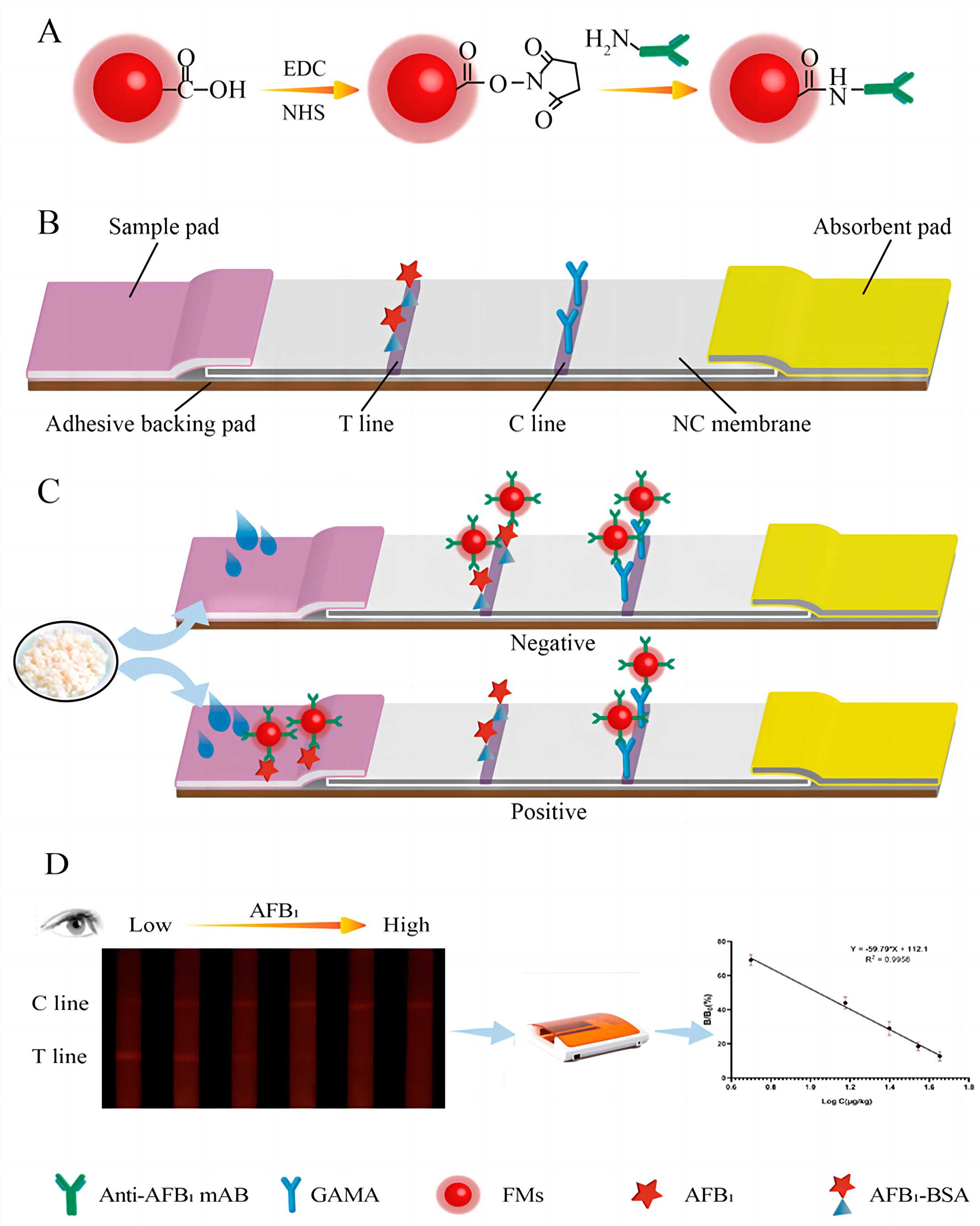 Fig.1 The structure chart and principle of FM-LFIA test strip.1, 2
Fig.1 The structure chart and principle of FM-LFIA test strip.1, 2
Comparison of FM-ICTS and CG-ICTS
CG-ICTS (colloidal gold immunochromatographic test strip) and FM-ICTS (fluorescent microsphere immunochromatographic test strip) are two common lateral flow immunoassays that utilize antigen-antibody properties and provide rapid detection of analytes. The two methods have been widely applied in many fields such as medical diagnosis, food safety, animal health, agriculture, and environmental science. In 2014, researchers developed FM-ICTS and CG-ICTS for the detection of Escherichia coli O157:H7 based on the sandwich format and compared the two test strips. Results showed FM-ICTS has advantages over CG-ICTS in terms of coupling rate, sensitivity, coefficient of variation, and antibody needed in a test. The sensitivity of FM-ICTS for E. coli O157:H7 detection in milk was 104 CFU/mL while the CG-ICTS was 105 CFU/mL.
FM-ICTS-based Detection of PRRSV Antibodies
PRRSV (porcine reproductive and respiratory syndrome virus) is the pathogen that causes PRRS. PRRSV infections cause reproductive failure in pregnant sows, respiratory distress in piglets and growing pig populations, and a delayed and inadequate adaptive immune response. Researchers have developed a rapid, ultra-sensitive, and quantitative FM-ICTS for the detection of PRRSV antibodies at the pen-side. This assay was based on the formation of a sandwich anti-pig IgG-PRRSV antibodies-NSP7/N complex. This assay has been validated as a promising pen-side diagnostic tool for detecting antibodies against the PRRSV with diagnostic specificity, sensitivity, and accuracy of 97.28, 93.41, and 94.95%, respectively.
Our Capabilities
With years of experience in IVD testing development, Creative Biolabs is confident in providing one-stop fluorescent microsphere-based lateral immunochromatographic assay development services for global clients. Our services include experimental scheme design, preparation of testing materials, validation of sensitivity, specificity, and stability of the testing. We guarantee to deliver the best assays with sensitivity, accuracy, and flexibility to support your IVD product in clinical application.
 Fig.2 Workflow of development of FM-ICTS.
Fig.2 Workflow of development of FM-ICTS.
If you are interested in our services, please contact us to discuss your project and achieve more details. We are your reliable partner to develop novel specific IVD assays for disease diagnosis research.
Published Data
1. Development of a Novel Time-Resolved Fluorescence Microsphere-Lateral Flow Immunochromatographic Strip for Detecting Pregnanediol-3-glucuronide in Urine Samples
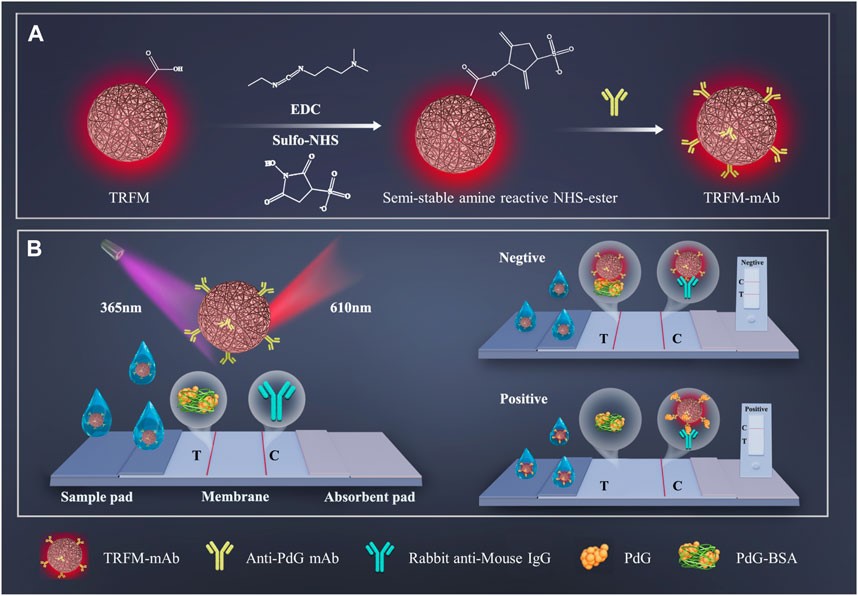 Fig.3 The schematic illustration of the preparation process and quantitative detection process of the TRFM-ICTS strip.3,2
Fig.3 The schematic illustration of the preparation process and quantitative detection process of the TRFM-ICTS strip.3,2
In this study, researchers successfully developed a competitive model-based time-resolved FM-ICTS (TRFM-ICTS) strip for rapid, sensitive detection of Pregnanediol-3-glucuronide (PdG), a key metabolite of progesterone in urine. They optimized the preparation parameters of the anti-PdG monoclonal antibody labeled with the TRFM probe and the TRFM-ICTS strip, followed by an evaluation of the strip's performance in terms of specificity, sensitivity, stability, precision, and recovery. The TRFM-ICTS strip showed a strong response to PdG within the range of 30–2,000 ng/mL, with a detection limit (LOD) as low as 8.39 ng/mL. When applied to urine samples, the strip demonstrated excellent recovery (97.39% to 112.64%) and strong sensitivity and selectivity. This strip holds significant potential for accurately detecting PdG in urine and can provide reliable predictions of ovulation periods.
References
- Wang, Zifei, Pengjie Luo, and Baodong Zheng. "A rapid and sensitive fluorescent microsphere-based lateral flow immunoassay for determination of aflatoxin B1 in distillers’ grains." Foods 10.9 (2021): 2109.
- Distributed under Open Access license CC BY 4.0, without modification.
- Lin, Jiasheng, et al. "A time-resolved fluorescence microsphere-lateral flow immunochromatographic strip for quantitative detection of Pregnanediol-3-glucuronide in urine samples." Frontiers in Bioengineering and Biotechnology 11 (2023): 1308725.
For Research Use Only.