Biomarker analysis services are utilized extensively during the earlier stages of the drug discovery process, primarily for mechanistic or pharmacodynamic purposes. This includes precisely determining a drug's mechanism of action (MOA), rigorously validating potential drug targets, closely monitoring therapeutic responses, and effectively guiding compound selections. Consequently, advanced biomarker analysis services provide crucial insights, helping to decide whether potential therapeutic agents should be efficiently discontinued or strategically pursued further in development.
Creative Biolabs offers unparalleled biomarker analysis services through a network of seasoned experts. We understand the multifaceted nature of biomarker selection, validation, and application from early drug discovery to in vitro diagnostics (IVD), supporting crucial decision-making and mitigating risk at every stage of your program. Creative Biolabs is exceptionally positioned to assist you with customized biomarker solutions for all your sample analysis needs.
Biomarker Analysis
A biomarker is fundamentally defined as a characteristic that can be objectively tested and assessed as an indicator of various biological processes. These processes can include normal physiological states, pathogenic conditions, or specific pharmacological responses to therapeutic interventions. Biomarker assays and the analytical data derived from them represent essential tools within the dynamic field of drug discovery, ranging from initial target validation studies to the critical process of candidate selection.
Importance of Biomarker Analysis
Objects for Biomarker Detection
Although any molecule that consistently reflects a specific biological state can be considered a biomarker, proteins are typically the most important and useful in life science research. The most common and effective strategy for comprehensive protein biomarker analysis involves the strategic use of commercially available kits. However, Creative Biolabs is also highly experienced in meticulously developing and tailoring biomarker assays specifically for our customers' unique requirements. There are two primary formats for biomarker analyses: Singleplex and Multiplex assays. Although single-analyte commercial kits are often very useful, their specificity, sensitivity, and availability can vary significantly. Creative Biolabs possesses extensive experience with a broad array of biomarkers from numerous kits and reagent vendors, enabling us to precisely identify the best option(s) for your specific biomarker project.
 Fig.1 Some hot research targets.
Fig.1 Some hot research targets.
Our Biomarker Testing Services
 Fig.2 Major analytical services at Creative Biolabs.
Fig.2 Major analytical services at Creative Biolabs.
 Fig.3 Platforms at Creative Biolabs.
Fig.3 Platforms at Creative Biolabs.
Service Workflow
We begin with a thorough initial consultation to deeply understand your specific research objectives, project requirements, and the nature of the starting materials you will provide. This ensures complete alignment on goals, expected outcomes, and necessary sample preparation.
Next, our expert team meticulously designs and develops fit-for-purpose assays, carefully selecting the most appropriate platforms and methodologies. This phase is tailored to precisely meet the analytical needs of your specific samples and biomarkers.
This crucial phase involves rigorous assay validation and qualification to ensure sensitivity, specificity, and reproducibility. All procedures are conducted in strict adherence to stringent regulatory standards, guaranteeing data integrity.
Subsequently, we proceed with the sample analysis, carefully processing your valuable biological samples using our cutting-edge instrumentation and proven protocols. This step ensures precise and reliable data acquisition.
Finally, we provide detailed data analysis and interpretation. This includes delivering comprehensive reports and actionable insights that empower your decision-making processes, translating complex data into clear scientific conclusions.
We ensure the final delivery of your comprehensive results and reports. Additionally, we offer continued post-delivery support, ensuring you have all the necessary understanding and assistance for your ongoing research.
Published Data
1. Label-Free LC-MS/MS Proteomic Analysis of Urine for Breast Cancer Biomarker Discovery
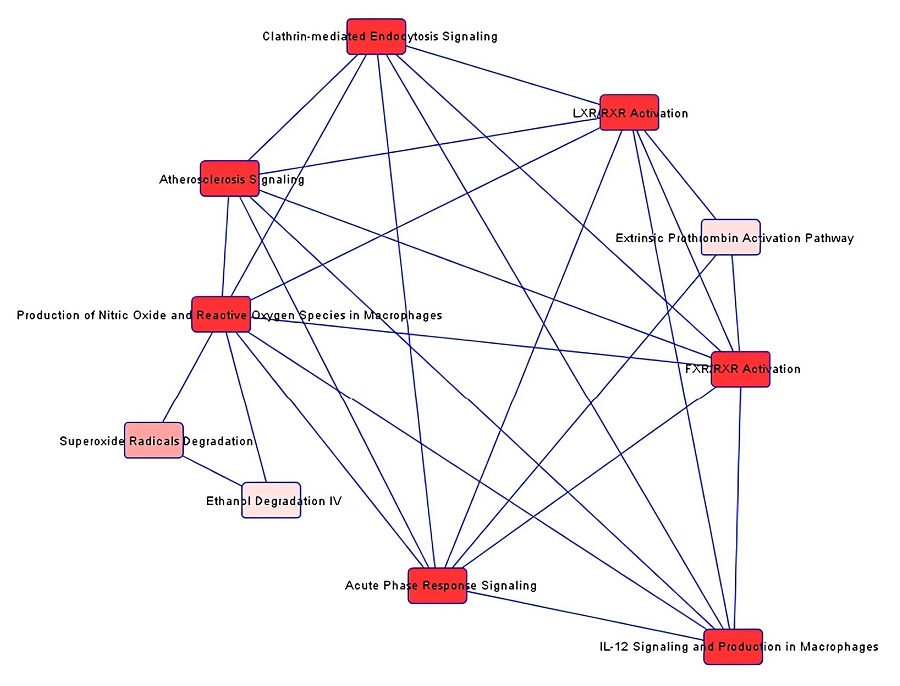 Fig.4 The interactive networks of breast cancer urinary proteins analyzed with LC-MS/MS.1
Fig.4 The interactive networks of breast cancer urinary proteins analyzed with LC-MS/MS.1
This work used unbiased, label-free LC-MS/MS-based proteomics to profile abundant proteins in breast cancer patient urine. Data analysis identified 59 urinary proteins significantly altered in breast cancer patients compared to controls (p<0.05, fold change >3). Among these, 36 proteins were stage-specific of breast cancer, with 24 upregulated and 12 downregulated. Amongst the 59 significant urinary proteins, 13 novel upregulated proteins were identified as potential biomarkers for breast cancer. Preliminary validation of three markers (ECM1, MAST4, and filaggrin) was conducted in breast cancer cell lines via Western blotting. The MAST4 biomarker was further validated in human breast cancer tissues and urine samples using immunohistochemistry and Western blotting, respectively.
2. Development of a Multiplex Immunoassay of Serum Biomarkers Aiding in Uveal Melanoma Detection
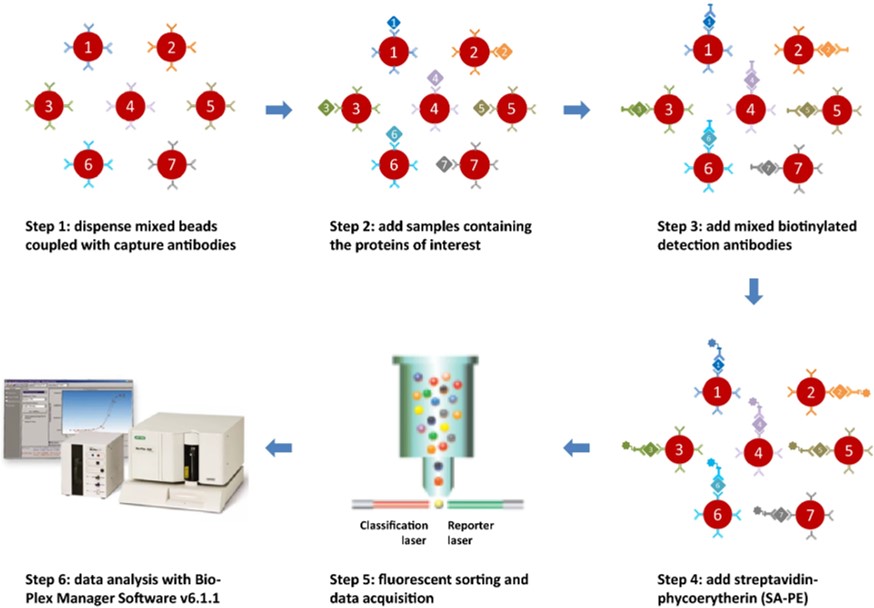 Fig.5 Workflow for magnetic bead-based multiplex immunoassay.2
Fig.5 Workflow for magnetic bead-based multiplex immunoassay.2
Researchers developed a magnetic bead-based 7-plex immunoassay to evaluate serum biomarkers for early uveal melanoma (UM) detection. The assay targeted CEACAM-1, OPN, POSTN, MIA, MIC-1, SPON1, and HSP27, showing minimal cross-reactivity, recovery rates between 84% and 105%, and intra-assay/inter-assay precision ranging from 2.3% to 7.5% and 2.8% to 20.8%, respectively. Serum samples from 48 UM patients, comprising 14 with metastatic disease, 9 disease-free, and 25 with an unknown status, along with 36 healthy control individuals, were evaluated. Logistic regression analysis revealed a two-marker combination of HSP27 and OPN, which enhanced the discriminatory ability of individual biomarkers. This multiplex immunoassay provides reliable analytical performance for detecting UM through complementary serum biomarkers.
Service Highlights
- Unrivaled Scientific Expertise: Our team comprises highly experienced biologists and bioinformaticians with deep therapeutic area knowledge. This ensures intelligent study design and insightful data interpretation for your projects.
- End-to-End Solutions: We provide seamless, integrated support across all phases of your research pipeline. Our services span from exploratory discovery to full analytical and clinical validation.
- Superior Data Quality: We adhere to rigorous quality control measures and employ highly sensitive, validated assays. This approach provides reliable, reproducible, and robust data you can trust for critical decisions.
- Customized & Flexible Approaches: Our services are meticulously tailored to your specific research needs. We offer bespoke assay development and analytical strategies to fit unique project requirements.
FAQs
-
What types of biological samples can you analyze for biomarker detection?
Creative Biolabs is equipped to analyze a wide array of biological matrices for biomarker detection. We routinely process samples, including but not limited to serum, plasma, urine, cerebrospinal fluid (CSF), and various tissue biopsies. Our advanced platforms are adaptable to diverse sample types, ensuring comprehensive analysis for your specific research needs.
-
How do you ensure the accuracy and reliability of its biomarker assays?
We ensure accuracy and reliability through a rigorous, multi-faceted approach. We implement strict quality control measures, adhere to industry-recognized validation guidelines, and utilize highly sensitive and specific detection technologies. Our assays undergo comprehensive analytical validation, guaranteeing robust and reproducible data for all client projects.
-
Can you develop custom biomarker assays for novel targets?
Indeed, we are experts in creating unique biomarker tests for new targets, and our skilled scientific staff collaborates directly with customers to create, refine, and validate custom assays. We leverage our extensive expertise and cutting-edge platforms to create assays tailored to your unique research requirements.
-
What turnaround times can I expect for biomarker analysis results?
Turnaround times for biomarker analysis results at Creative Biolabs vary depending on the complexity and volume of the project. We are committed to efficient processes and timely delivery, and a detailed timeline will be provided during the initial consultation. Our optimized workflows are designed to meet critical milestones effectively.
-
How do you handle data analysis and interpretation after biomarker testing?
We provide meticulous data analysis and interpretation following biomarker testing. Our in-house bioinformatics and biostatistical experts transform raw data into actionable insights, utilizing advanced computational tools and machine learning algorithms. We deliver comprehensive reports that are clear, concise, and directly applicable to your research decisions.
-
Is Creative Biolabs able to support both singleplex and multiplex biomarker assays?
Yes, Creative Biolabs is fully equipped to support both singleplex and multiplex biomarker assays. We offer expertise in developing and running both formats, allowing for precise quantification of individual analytes or simultaneous measurement of multiple biomarkers from a single sample. This flexibility caters to diverse research needs.
Our available services can function as a standalone offering or be integrated into an end-to-end preclinical program. Creative Biolabs employs both traditional and novel platforms, as well as ultra-sensitive detection methods, including single and multiplex analysis, to support diverse research fields. We are proud to become a benchmark in the field and significantly impact clients by advancing drug development. If you are interested in our biomarker analysis services, please feel free to contact us for more information.
References
- Beretov, Julia, et al. "Proteomic analysis of urine to identify breast cancer biomarker candidates using a label-free LC-MS/MS approach." PloS one 10.11 (2015): e0141876. Distributed under Open Access license CC BY 4.0, without modification.
- Song, Jin, et al. "A multiplex immunoassay of serum biomarkers for the detection of uveal melanoma." Clinical Proteomics 16 (2019): 1-13. Distributed under Open Access license CC BY 4.0. The image was modified by extracting and using only part of the original image.
For Research Use Only.