Developing specialized assays is critical for high-throughput screening and lead optimization. These assays can be designed to detect compound-target interactions, measure cellular responses, or evaluate the potency and selectivity of novel drug candidates, dramatically accelerating the drug discovery pipeline.
Creative Biolabs' custom assay development services are meticulously designed to bridge the path from discovery to a validated product. Our scientific expertise delivers highly tailored solutions for unique in vitro diagnostic (IVD) assays, meeting the diverse needs of our clients in basic, applied, and clinical research. This precision is the key to accelerating innovation.
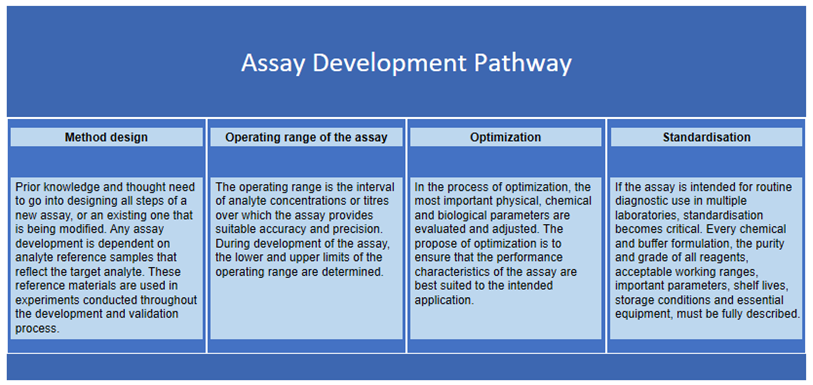
Assay Development
The initial phase of developing a robust diagnostic assay requires demonstrating its feasibility—the ability to detect a specific analyte under optimized conditions. This is followed by a rigorous evaluation of key analytical metrics. The development process meticulously determines analytical sensitivity, analytical selectivity, limit of detection (LoD), trueness, and precision. These crucial statistics provide an abundance of information, revealing the assay's quantitative response to varying analyte amounts, its reliable reportable range, and its linear behavior. Potential interferents are also assessed to ensure the assay is both accurate and reliable for its intended purpose.
Custom Assay Development Services at Creative Biolabs
With years of experience in IVD assay development, Creative Biolabs has a full spectrum of capabilities for a wide range of IVD assay development, including enzyme-linked immunosorbent assay (ELISA)-based assay, radioImmunoAssay (RIA)-based assay, lateral-flow immunochromatographic assay (LFIA)-based assay, latex particle-enhanced turbidimetric immunoassay (LETIA)-based assay, western blot (WB)-based assay, immunohistochemistry (IHC)-based assay, and flow cytometry (FC)-based assay. We have the expertise to design, develop and execute the necessary assays at all phases of the project. Our development process is the fastest, high-efficient and most streamlined system to get our clients' IVD assay project through our proven quality control system, manufactured, approved and to market. Our seasoned scientists can direct and manage all aspects of the assay efforts and the resulting data that is generated. By applying our staff's experience and a proven quality system, we are confident to complete your IVD assay development project with speed, accuracy and the quality necessary for international regulatory agency approvals.
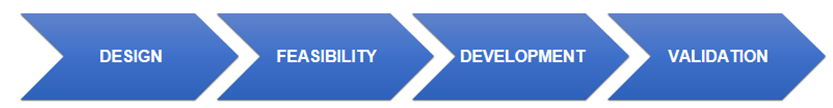
Service Workflow of Custom Assay Development
Our comprehensive project workflow is a structured, step-by-step process designed to ensure clarity and success from start to finish.
The project begins with a detailed discussion to define your research goals, technical specifications, and performance criteria. This collaboration results in a detailed Statement of Work (SOW), which acts as our shared blueprint.
Our team assesses the viability of the project and selects the most suitable technology platform and reagents. This phase ensures the assay concept is sound before proceeding.
This stage involves the iterative fine-tuning of the assay protocol. We conduct a series of experiments to optimize conditions, ensuring the assay achieves maximum sensitivity and specificity.
The developed assay is rigorously tested against the SOW. This step confirms that all performance metrics, from linearity to precision, are met and validated.
The project concludes with the delivery of a comprehensive technical report and a detailed protocol. We ensure a smooth transfer of the assay into your laboratory with ongoing support.
Applications
Drug Discovery & Screening
Biomarker Identification & Validation
The precise detection and quantification of novel biomarkers is essential for personalized medicine. Custom assays allow for the validation of new biomarkers in complex sample matrices, providing a reliable tool for early disease detection, patient stratification, and monitoring therapeutic responses.
Companion Diagnostics
For a new therapeutic to be successful, a companion diagnostic assay is often needed to identify which patients will respond. We develop custom IVD assays that are optimized for clinical use and adhere to the strict regulatory standards required to support drug submissions.
Basic & Applied Research
Researchers working on unique biological systems or with rare analytes require tailor-made solutions. Our services enable the creation of highly specific research tools, from cell-based functional assays to novel protein-protein interaction assays, that provide accurate and reproducible data.
Published Data
1. In Vitro Diagnostic Assay for SARS-CoV-2-Neutralizing Antibody Detection Using Engineered Mini-Protein
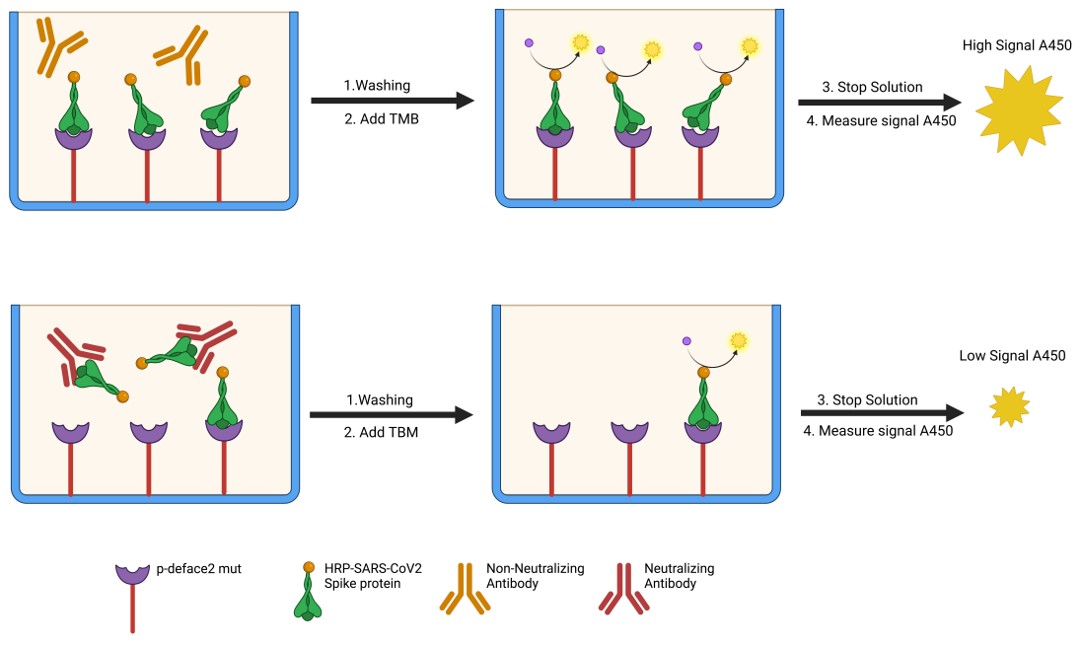 Fig.1 The IVD assay based on engineered mini-protein for detecting SARS-CoV-2 NAbs.1
Fig.1 The IVD assay based on engineered mini-protein for detecting SARS-CoV-2 NAbs.1
This study introduced an engineered mini-protein, p-deface2 mut, to develop an in vitro diagnostic (IVD) assay based on the competitive ELISA, where the ACE2 protein in the test kit was replaced with p-deface2 mut protein. The assay helped to detect SARS-CoV-2 neutralizing antibodies (NAbs) in patient sera and determine when a COVID-19 booster is necessary. Serum samples from 21 patients were tested, with 9 samples (42.8%) testing positive and 12 samples (57.1%) testing negative for NAbs. Results were consistent with those from a standard commercial assay using human ACE2 protein, confirming that p-deface2 mut can effectively replace ACE2 in ELISA testing. As the protein can be bacterially expressed, it offers a cost-effective alternative for large-scale NAb detection. This assay will assist healthcare providers in determining the need for boosters, reducing reinfection risks by ensuring vaccines are administered at the optimal time.
2. Development of a Rapid, Sensitive Point-Of-Care Testing for COVID-19 Diagnosis
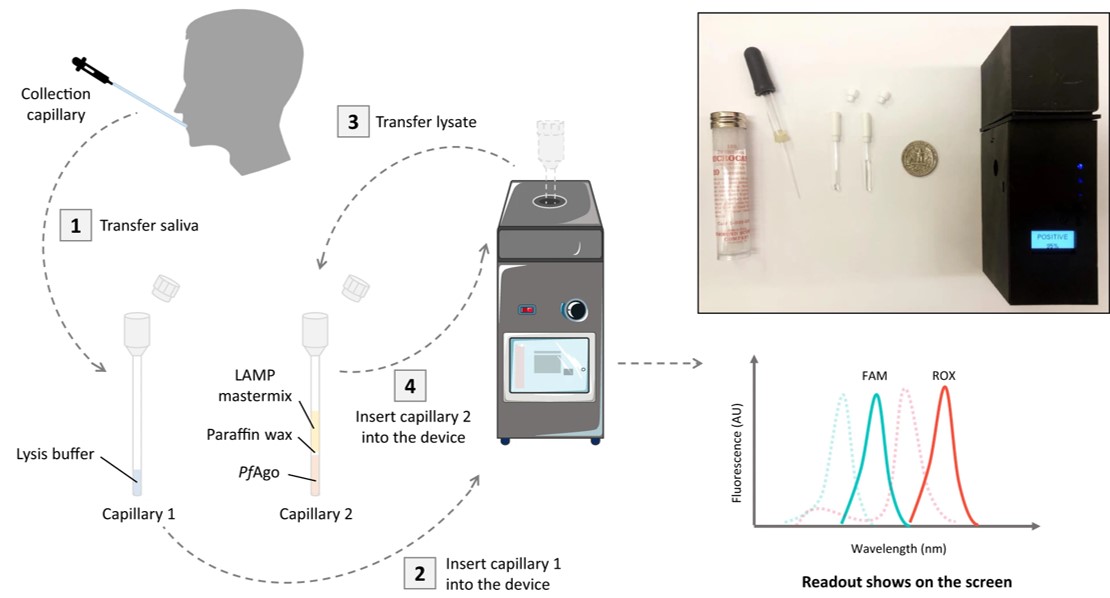 Fig.2 Overview of the SPOT system.2
Fig.2 Overview of the SPOT system.2
This work presented a rapid, scalable, and portable COVID-19 testing system (SPOT) that combined a highly sensitive assay with a battery-powered portable device. The SPOT assay used reverse transcriptase-loop-mediated isothermal amplification (RT-LAMP) combined with PfAgo-based detection to identify the N and E genes of SARS-CoV-2 in a multiplexed reaction. It achieved a limit of detection (LoD) of 0.44 copies/μL for the N gene and 1.09 copies/μL for the E gene in saliva samples, with results in 30 minutes. In clinical testing of 104 saliva samples, the system demonstrated 93.3% sensitivity and 98.6% specificity, offering a fast, accurate, and cost-effective solution for widespread COVID-19 testing.
Service Highlights
- In-Depth Knowledge & Expertise: Our team possesses deep domain knowledge in IVD assay development, leveraging a full spectrum of capabilities to address a wide range of scientific challenges with a robust and reliable approach.
- Proven Technology: We utilize proven, first-in-class technology to power our assays, ensuring high-quality, dependable performance and generating results you can trust for your critical research.
- High Flexibility & Cost-Effectiveness: Our workflows are designed for maximum flexibility, allowing us to adapt to your unique project requirements. This, combined with our optimized processes, helps to significantly reduce development time and cost.
- Reliability: We adhere to a rigorous quality management system, ensuring all our assays are developed with a strong focus on reproducibility and consistent analytical performance, which is vital for regulatory submissions.
- Information Security: Protecting your valuable proprietary data is our top priority. We operate with strict confidentiality protocols and a secure development environment to safeguard your information throughout the entire development process.
FAQs
-
Q: What types of assay platforms do you have experience with?
A: Our expertise spans a wide array of platforms, including but not limited to ELISA, Western blot, IHC, and various cell-based and biochemical assay formats. During the initial consultation, we select the best platform based on your project's specific needs and performance requirements, ensuring a fit-for-purpose solution. We remain technology-agnostic to provide the most suitable approach for your specific research question.
-
Q: How do you ensure the confidentiality of my proprietary research?
A: Protecting your intellectual property is a top priority. We operate under strict confidentiality and non-disclosure agreements (NDAs) to safeguard your proprietary data and unique research materials throughout the entire development process. All project data is stored in a secure environment accessible only to authorized personnel.
-
Q: Can you work with my own proprietary antibodies or reagents?
A: Absolutely. Our collaborative approach means we can seamlessly integrate your own proprietary materials into the assay development workflow, optimizing them to ensure they perform effectively within the final solution. This not only leverages your existing assets but also helps to ensure continuity with your ongoing research efforts.
-
Q: What is the minimum sample volume required for a feasibility study?
A: The required sample volume varies significantly depending on the target analyte's concentration and the selected assay technology. This crucial detail will be determined and specified during the initial consultation and project design phase to ensure we have sufficient material without waste. Our team works to minimize material usage wherever possible while maintaining data integrity.
-
Q: How do you ensure the custom assay is reproducible?
A: Reproducibility is a key focus of our development process. We employ a rigorous, multi-stage validation workflow that includes testing against a comprehensive set of analytical metrics, ensuring the assay generates consistent and reliable results every time. This robust process is central to our quality management system and builds trust in the data produced.
-
Q: What support do you offer after the assay is delivered?
A: Our commitment to your success extends beyond the final delivery. We provide a comprehensive technical support package, including a detailed protocol, a final report, and access to our scientific experts for ongoing consultation to ensure seamless integration and long-term success. We are available to answer questions and troubleshoot any issues that may arise.
-
Q: What are the major factors that can influence the cost of custom assay development?
A: The cost is influenced by several factors, including the complexity of the target, the required performance characteristics, the specific technology platform chosen, and the number of optimization steps needed to achieve the desired result. The overall scope of work and the required validation level are also key cost drivers.
Scientists at Creative Biolabs are experts in IVD assay development. If you don't find the assay you want, bring your custom projects to us. Every assay development will require a custom design. We consult with you from start to finish to meet your unique needs. Please contact us to discuss your assay development needs and a detailed quote.
References
- Pereira de Jesus, Bruna Andersen, et al." In Vitro Diagnostic Assay to Detect SARS-CoV-2-Neutralizing Antibody in Patient Sera Using Engineered ACE-2 Mini-Protein." Viruses 14.12 (2022): 2823. Distributed under Open Access license CC BY 4.0, without modification.
- Xun, Guanhua, et al. "A rapid, accurate, scalable, and portable testing system for COVID-19 diagnosis." Nature communications 12.1 (2021): 2905. Distributed under Open Access license CC BY 4.0. The image was modified by extracting and using only part of the original image.
For Research Use Only.