Rapid, efficient and accurate nucleic acid molecule detection is important in the screening of diseases and pathogens. Microfluidic systems are characterized by fast, integrated, miniaturized features that provide an effective platform for qualitative and quantitative detection of nucleic acid molecules. With experience in Microfluidic design and advanced Microfluidic platforms, Creative Biolabs is competent to offer the best-qualified nucleic acid detection and analysis services based on Microfluidic technology to meet our customers' specific requirements.
Integration of Nucleic Acid Detection and Microfluidics
To provide real-time diagnosis of diseases and early screening for diseases, it is important to develop techniques that allow for accurate and rapid detection of nucleic acids. Differentiating by temperature control conditions, nucleic acid amplification techniques can be divided into isothermal and non-isothermal amplification techniques. Multiplex PCR assays, DNA microarrays, and various nanotechnology-based nucleic acid sensors are examples of major techniques developed for analyzing nucleic acids. However, these methods have several challenges such as lengthy preparation and analysis time, vulnerability to contamination, complex procedures and operation, and sensitivity limitations. The challenges limit its effectiveness in point-of-care (POC), especially in resource-limited settings. Integration of Microfluidic technology with nucleic acid extraction helps to address these technical and analytical limitations associated with nucleic acid detection.
Detection Approaches
Microfluidics-based approaches provide an ideal platform for nucleic acid amplification and rely mainly on fast prototyping by soft lithography in polydimethylsiloxane (PDMS) or glass. In recent years, many kinds of nucleic acid amplification approaches such as PCR and isothermal amplification have been performed on the Microfluidic system with the achievement of fast and accurate detection.
- Digital PCR (dPCR) amplification of nucleic acids
qPCR is a relative quantification technique that relies on a standard curve or reference gene assessment to determine the amount of target nucleic acid. For low copy number target analyte, issues such as primer efficiency and differences in template concentrations can markedly affect the detection sensitivity and accuracy at the end-stage.
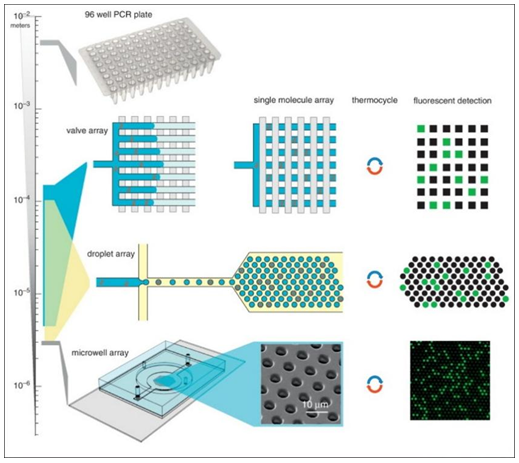 Fig.1 Microfluidic digital PCR.1
Fig.1 Microfluidic digital PCR.1
- Isothermal quantification of nucleic acids
The ease of operation, high specificity and high sensitivity make nucleic acid isothermal amplification a potential tool for POC. The nucleic acid isothermal amplification technology does not require different temperature cycles to generate new products from the nucleic acid template. It does not depend on sophisticated equipment and has shown good application prospects in POC. Among various isothermal amplification techniques, loop-mediated isothermal amplification (LAMP), recombinase polymerase amplification (RPA), and rolling circle amplification (RCA) has attracted significant attention from the Microfluidics community.
Application Examples
Microfluidics is increasingly being explored and validated for use in the detection and diagnosis for cancers from colorectal carcinoma, hepatocellular carcinoma, to prostate cancer and different types of infectious diseases from food-borne pathogen and Hepatitis B to meningitis and dengue virus, and other diseases from cardiovascular disease to Alzheimer's diseases.
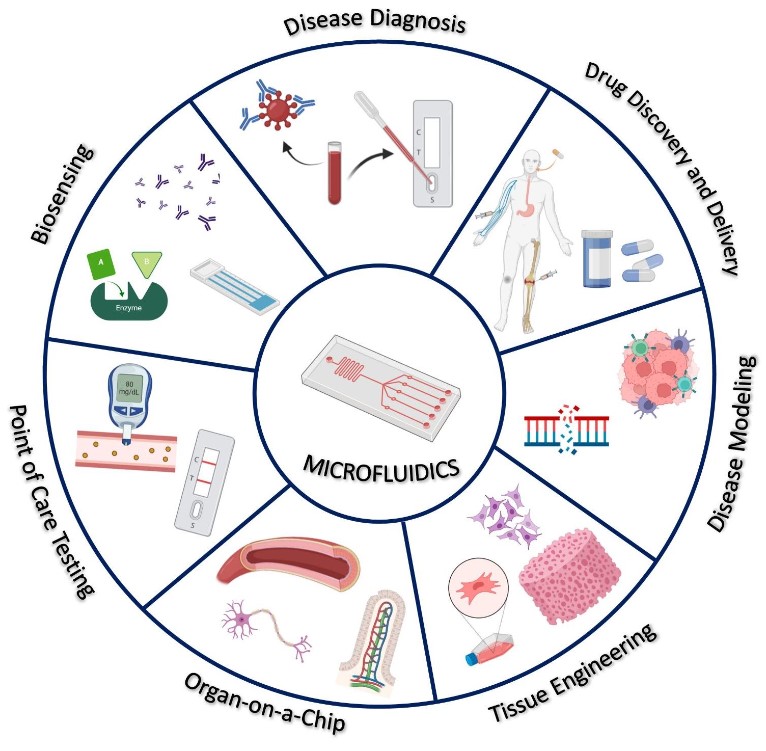 Fig.2 Biomedical applications of microfluidic devices.2, 4
Fig.2 Biomedical applications of microfluidic devices.2, 4
Visual detection of pathogen nucleic acids also has broad applications in the detection of pathogen nucleic acids. For instance, an enzyme-assisted nano-complexes for visual identification of nucleic acids has been reported. The system consists of an integrated circuit of enzyme-DNA nanostructures, which function as independent recognition and signaling elements, for direct and versatile detection of pathogen nucleic acids from infected cells. When implemented on a configurable Microfluidic platform, the technology demonstrates superior programmability to perform versatile computations, for detecting diverse pathogen targets and their virus-host genome integration loci.
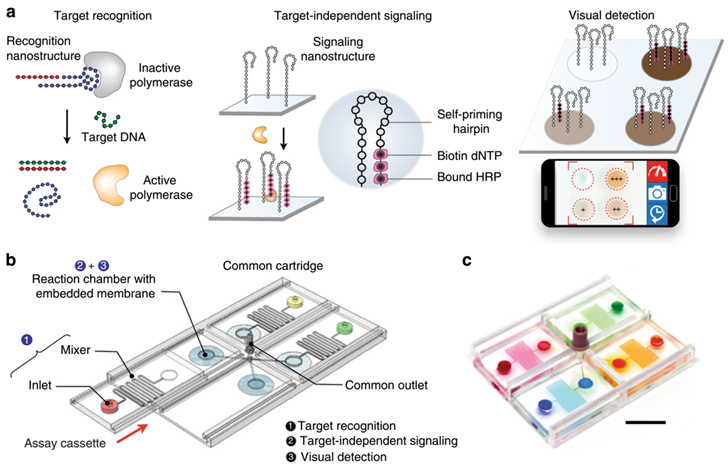 Fig.3 Visual and modular detection of pathogen nucleic acids.3, 4
Fig.3 Visual and modular detection of pathogen nucleic acids.3, 4
Featured Services
Creative Biolabs now offers Microfluidics-based nucleic acid detection and analysis services, including but not limited to:
- Detection of nucleic acids on Microfluidic chips
- Isothermal amplification on Microfluidic chips
- Non-isothermal amplification on Microfluidic chips
- Digital amplification on Microfluidic chips
- Tailed signal read-out
- Different types of detection methods, including electrophoresis separation, optical density, fluorescence signals, turbidity and by electrochemical sensing.
- Other gene-level detection services
Advantages
- Labs on a chip allow for the integration of sample preparation, amplification, and signal detection, reducing the time need to generate results.
- Microfluidic chip enables improved efficiency in analyzing nucleic acids to evaluate the existing state of a specific gene and detailed information such as single nucleotide polymorphism (SNP), insertion, and deletion.
- LAMP-based quantification of nucleic acids
- RCA-based quantification of nucleic acids
- Padlock probe with RCA on Microfluidic systems
- Multiple sample preparation methods based on silica beads, silicon micropillars, membrane, paper and magnetic beads
- Tailored service according to your project need
With the high demand for rapid detection tools for diseases and pathogens in the fields of disease diagnosis, the focus has been directed towards molecular diagnostic approaches for POC. By virtue of its own technical advantages, Microfluidic systems have been developed and integrated to perform nucleic acid detection for diverse application. Creative Biolabs also provides Microfluidic-based POCT Development Services for global clients. If you are interested in our services or seeking cooperation, please feel free to contact us for more information.
Published Data
1. An Integrated Microfluidic Platform for Nucleic Acid Testing
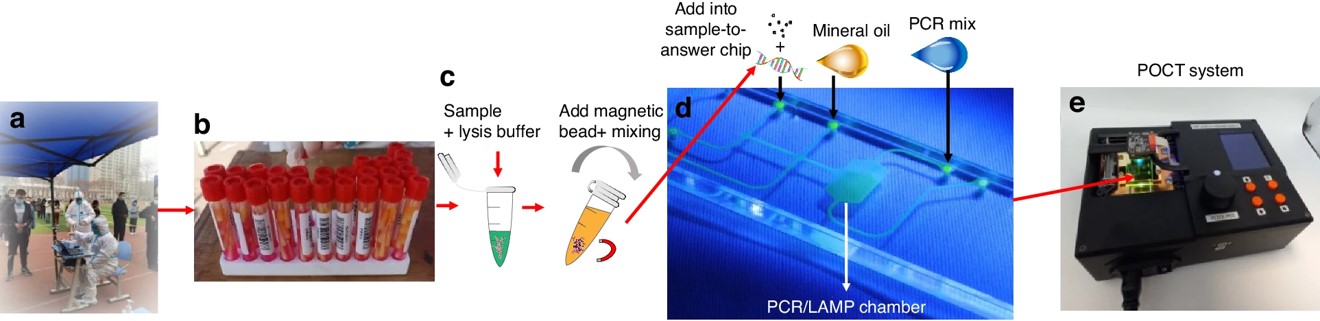 Fig.4 Workflow of a microfluidic diagnostic system for RNA detection.5,4
Fig.4 Workflow of a microfluidic diagnostic system for RNA detection.5,4
This study presented a low-cost, rapid, and versatile sample-to-answer system for SARS-CoV-2 diagnostics, integrating nucleic acid extraction and purification with amplification via reverse transcription-quantitative PCR (RT-qPCR) or reverse transcription loop-mediated isothermal amplification (RT-LAMP). The platform delivered results that closely matched those of commercial RT-LAMP and RT-qPCR systems, offering advantages in speed and cost-effectiveness. The assay was completed in 28 minutes, including sample loading (5 minutes), RNA extraction (3 minutes), and RT-LAMP (20 minutes), with a cost of approximately $9.50 per test, competitive with FDA-approved commercial alternatives. Despite some RNA loss during on-chip extraction, the platform maintained a detection limit below 297 copies. Its portability makes it ideal for environments without centralized laboratories, and users can choose between RT-qPCR or RT-LAMP based on specific needs.
2. Development of a Passively Driven Microfluidic Device for Simple and Efficient Nanoliter Droplet-Based Nucleic Acid Detection
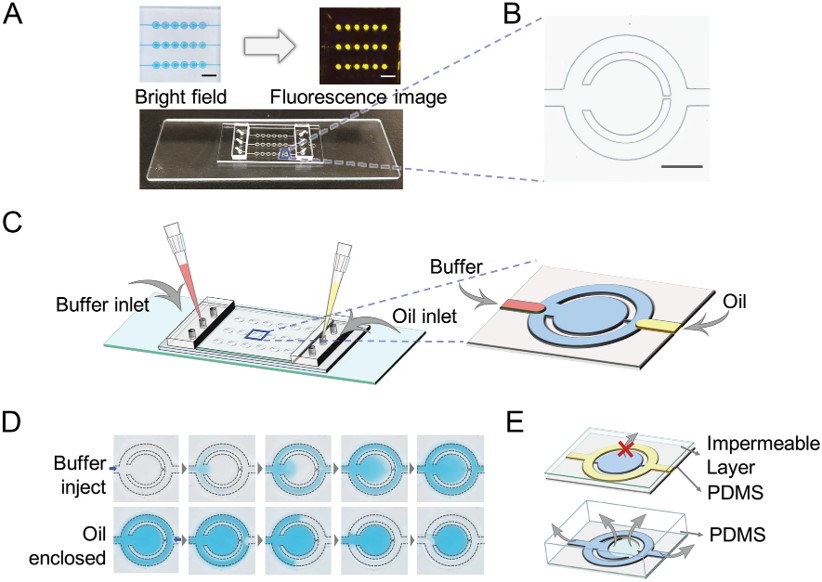 Fig.5 Schematic illustration of the passive-driven microfluidic-LAMP chip.6,4
Fig.5 Schematic illustration of the passive-driven microfluidic-LAMP chip.6,4
This study demonstrated a simple, valve-free, and pump-free microfluidic system, offering consistent nucleic acid analysis from 18 uniform droplets using LAMP detection. Users could precisely control the nanoliter-scale droplets in the system by utilizing only micropipette regulation. The use of an oil enclosure and impermeable fabrication helped preserve reagents, reducing fluid loss and preventing condensation. The fluorescence intensity's relative standard deviation (RSD) was <5% between droplets and <2.0% during heating. Besides, the microfluidic-LAMP chip exhibited broad applicability for both genome detection and gene expression analysis across different nucleic acid detection methods.
References
- Streets, Aaron M., and Yanyi Huang. "Microfluidics for biological measurements with single-molecule resolution." Current opinion in biotechnology 25 (2014): 69-77. Distributed under Open Access license CC BY 3.0, without modification.
- Gharib, Ghazaleh, et al. "Biomedical applications of microfluidic devices: a review." Biosensors 12.11 (2022): 1023.
- Ho, Nicholas RY, et al. "Visual and modular detection of pathogen nucleic acids with enzyme–DNA molecular complexes." Nature communications 9.1 (2018): 3238.
- Distributed under Open Access license CC BY 4.0, without modification.
- Sun, Antao, et al. "An integrated microfluidic platform for nucleic acid testing." Microsystems & Nanoengineering 10.1 (2024): 66.
- Lin, Pei-Heng, and Bor-Ran Li. "Passively driven microfluidic device with simple operation in the development of nanolitre droplet assay in nucleic acid detection." Scientific reports 11.1 (2021): 21019.
For Research Use Only.