Organ-on-a-Chip Basics
Organ-on-a-chip (OOC) is a relatively new technology in biomedical research which aims to develop the best ways to mimic microenvironment and physiological function of human organs in vitro. These "chips" are not the usual microprocessors that are made out of silicon, but instead, they are carefully engineered and flexible thumb-drive sized devices that integrate advanced cell culture techniques with gut microfluidics. The objective of these microchips is to create miniaturized, functioning models of human organs to use them as more human-relevant and predictive platforms for various purposes. The OOC technology fills the long existing gap between the oversimplification of 2D cell cultures in petri dishes and the ethical, physiological and translational limitations of animal models.
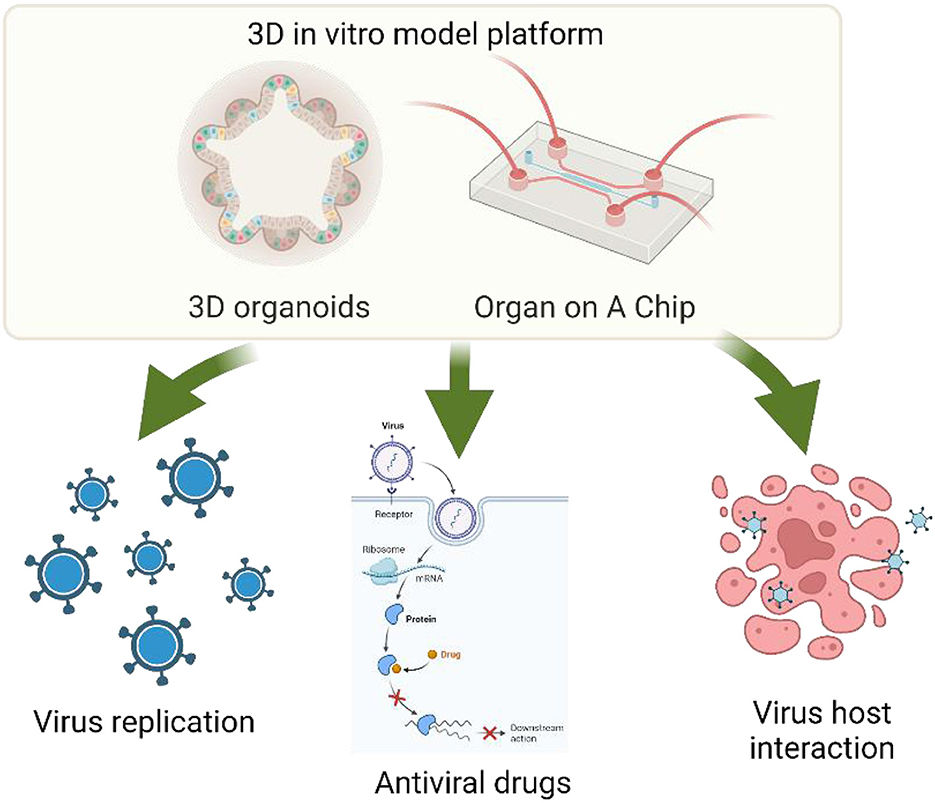 Figure 1 3D organoid applications for virology and antiviral research.1
Figure 1 3D organoid applications for virology and antiviral research.1
The Core Components of Organ-on-a-Chip
Every organ-on-a-chip device integrates key components crucial for recreating a living organ's complexity:
-
Microfluidic Channels: Gut Channels as small as tens to hundreds of micrometers in diameter can be formed inside biocompatible polymers, such as PDMS, as miniaturized vascular networks that can ensure media containing nutrients and oxygen are continuously and accurately provided to cells and that waste products are efficiently removed. At this scale, laminar flow can provide stable concentration gradients mimicking the physiological state.
-
Cell Chambers: These are microfluidic compartments that are loaded with organ-specific cells. The cell self-assembly environment within the chamber creates a three-dimensional arrangement of cells (e.g. alveolar sacs in a lung-on-a-chip, villi in a gut-on-a-chip). This 3D arrangement is necessary for simulating the 3D cell-cell and cell-ECM interactions that occur in the native organ, and ultimately the emergent tissue-level functionality that would be impossible in 2D cultures.
-
Porous Membrane: A thin, flexible, and porous membrane often separates the parallel microfluidic channels. This membrane serves as an interface between different cellular compartments. It is coated with extracellular matrix (ECM) material to facilitate cell adhesion and growth. Its porosity allows selective transport of molecules, nutrients, and signaling factors between the compartments. It also mimics the physiological barriers and pathways for cell-cell communication in the body (e.g. the air-blood barrier in the lung). Its flexibility is also important for applying mechanical forces.
Organ-on-a-Chip Workflow: A Step-by-Step Approach
Developing and utilizing an Organ-on-a-Chip involves several critical stages, each contributing to the faithful replication of human physiological systems:
01 Design & Fabrication
The microfluidic chip is designed based on the desired tissue/organ's characteristics. 3D printing or soft lithography are two of the most common techniques to achieve this. The channels and the flow of culture medium are carefully designed to match the structure and function of the desired tissue/organ.
|
Technical Method
|
Principle
|
Applicable Scenarios
|
|
Photolithography
|
UV light exposes photoresist through a mask, followed by etching silicon molds and PDMS injection molding.
|
High-precision structures (e.g., vascular networks)
|
|
3D Bioprinting
|
Layer-by-layer deposition of bio-inks (cell-laden hydrogels) to directly construct complex cellularized structures.
|
Personalized organ models (e.g., tumor-on-a-chip)
|
|
Molding
|
Low-cost mass production; however, PDMS may adsorb hydrophobic molecules, potentially affecting drug testing.
|
High-throughput screening
|
Target cells (e.g. endothelial, epithelial) are introduced into designated regions of the chip. Cells multiply and differentiate into tissue-like constructs within the microfluidic system, requiring specific media and growth factors for viability and function over a period of days to weeks.
03 Microenvironment Simulation
In order to recreate an in vivo environment, the chip is maintained in a precisely controlled setting. This includes managing the flow of fluid, pH, nutrient levels, and shear force, allowing for expression of physiological functions such as intercellular signaling and tissue repair.
04 Data Acquisition & Analysis
Real-time monitoring techniques, such as microscopic imaging, electrophysiological recording, and biosensors, are utilized during the culture period. The acquired data can be utilized to evaluate cellular function, drug response, and disease models.
With the organ model established, the next step is to use it for drug screening. Drugs are added at controlled concentrations and exposure times to observe how they affect cell function and to assess drug efficacy and toxicity.
06 Multi-Organ Integration
Chips representing different organs can be interconnected to produce a "Body-on-a-Chip". This allows for a more accurate representation of human physiology, allowing for the study of inter-organ interactions.
 Figure 2 Organ-on-a-Chip Workflow. (Creative Biolabs Original)
Figure 2 Organ-on-a-Chip Workflow. (Creative Biolabs Original)
Why Organ-on-a-Chip Outshines Traditional Models?
|
Feature
|
Organ-on-a-Chip (OOC)
|
Traditional 2D Cell Culture
|
Animal Models
|
|
Physiological Relevance
|
High: Mimics 3D structure, dynamic forces, flow.
|
Low: Flat monolayer, static.
|
Variable: Species differences limit human relevance.
|
|
Reproducibility/Control
|
High: Precise control, consistent conditions.
|
High: Easy to replicate basic setup.
|
Moderate to Low: High variability, difficult to control.
|
|
Cost
|
Moderate to High (initial); potentially lower long-term.
|
Low.
|
High.
|
|
Human Relevance
|
High: Uses human cells.
|
Low: Often uses standardized cell lines.
|
Limited: Physiological differences.
|
|
Predictivity for Humans
|
High: Better translation to human responses.
|
Low: Poor translation.
|
Moderate: Limited by species differences.
|
|
Ethical Considerations
|
High: Reduces animal testing.
|
High: No animal involvement.
|
Low: Involves sentient animals.
|
|
Drug Testing Speed
|
Faster: Accelerates preclinical testing.
|
Faster: Quick basic experiments.
|
Slower: Long durations, approvals.
|
|
Personalization
|
High: Patient-derived models possible.
|
Low: Limited personalization.
|
Low: No specific patient response testing.
|
|
Microenvironment Control
|
Excellent: Precise control of all factors.
|
Limited: Static, lacks dynamic control.
|
Limited: Hard to control in vivo.
|
|
Real-time Monitoring
|
High: Transparent, integrated sensors.
|
Moderate.
|
Low: Difficult, often invasive.
|
What Promising Horizons Await Organ-on-a-Chip Technology?
The future of organ-on-a-chip models appears promising. Organ-on-a-chip models are anticipated to be used to make drug screening models more predictable, accelerating the drug development process. Another potential application of organ-on-a-chip models is personalized medicine. Patient - derived cells could be used to make a drug for a particular individual based on their genetic and physiological information. The use of artificial intelligence and machine learning with organ-on-a-chip models is also a possibility, enabling data analysis and model optimization. Finally, multi - organ-on-a-chip models are anticipated to become more prevalent as the technology continues to evolve.
Frequently Asked Questions
Q: How accurate are Organ-on-a-Chip models in predicting human responses to drugs?
A: While organ-on-a-chip models have yet to match human drug responses, they have shown considerable potential. Some studies have found that organ-on-a-chip models can outperform other models for drug metabolism and toxicity. They can mimic the interaction of cells, tissues, and microenvironments within an organ, and as the technology develops further, with improvements in cell culture techniques, microfluidic design, and multiple organ-on-a-chip units, they are expected to become even more predictive. Development of multi - organ-on-a-chip models, capable of modelling the interaction between different organs, is an especially promising field for increasing the accuracy of drug response prediction.
Q: Can Organ-on-a-chip models be used to study rare diseases?
A: Yes, organ-on-a-chip models can be used to study rare diseases. Since rare diseases typically affect specific organs or organ systems, the organ-on-a-chip model can be tailored to recapitulate the relevant pathological conditions. For example, if a rare disease is associated with a malfunctioning kidney, a kidney-on-a-chip model can be engineered to incorporate the genetic or cellular abnormalities associated with that disease. This allows researchers to study the disease mechanism in a more controlled environment, which is often difficult to do using traditional research methods due to the scarcity of patient samples. Additionally, organ-on-a-chip models can be used to screen potential drugs for rare diseases, which may speed up the development of new treatments.
Q: What are the limitations of current Organ-on-a-chip technology?
A: One of the downsides of the early organ-on-a-chip designs is that simulating an isolated organ may miss important biological processes that take place in the complex web of interactions that exists in the human body. This simplification limits the extent to which conclusions can be drawn from the data. Another issue is the challenge of accurately replicating all aspects of an organ's function. For example, some organs have very intricate 3D structures and cell-to-cell interactions that are difficult to mimic on a chip. There is also the issue of maintaining the long-term viability and stability of the cell cultures on the chip. Finally, the costs associated with designing and running organ-on-a-chip experiments can be relatively high, which may restrict their use in certain research environments. These challenges are currently being addressed through the development of more advanced multi-organ-on-a-chip models and improvements in cell culture techniques.
Overview of What Creative Biolabs Can Provide
Creative Biolabs is pioneering Organ-on-a-chip research and is committed to developing innovative solutions. Our team is composed of professional scientists and engineers who have expertise in developing microfluidic technologies and our wide range of function - proven 3D tissue and organ-on-a-chip models will facilitate your research.
Custom Organ-on-a-Chip Model Development:
At Creative Biolabs, we understand that each research question demands a tailored approach. Our offerings are designed to provide flexibility, precision, and human relevance, attracting customers seeking advanced preclinical models. Contact us today to learn more!
References
-
Yin Y, Xu L, Steinway S N. 3D organoid and organ-on-a-chip and their applications for virology and antiviral research. Frontiers in Microbiology, 2023, 14: 1266136. https://doi.org/10.3389/fmicb.2023.1266136. Distributed under Open Access license CC BY 4.0, without modification.
Research Model
Related Sections:

 Figure 1 3D organoid applications for virology and antiviral research.1
Figure 1 3D organoid applications for virology and antiviral research.1
 Figure 2 Organ-on-a-Chip Workflow. (Creative Biolabs Original)
Figure 2 Organ-on-a-Chip Workflow. (Creative Biolabs Original)