3D Spheroids Introduction
3D spheroids develop as spherical cellular masses that spontaneously self-assemble or form through directed assembly in specialized culture environments without cell adhesion. Cells in spheroids retain their natural polarity while establishing strong cell-cell junctions such as tight junctions and desmosomes and produce extracellular matrix components that create an in vivo-like microenvironment unlike the flattened cells in 2D monolayers on rigid substrates. The internal structure of spheroids demonstrates variable complexity based on the cell type and culture method, ranging from basic homogeneous clusters to intricate formations with differentiated layers like a proliferating outer layer and a necrotic center seen in larger tumor spheroids that resemble avascular tumors. Spatial heterogeneity affects how drugs penetrate cells as well as cell survival and gene expression patterns which positions spheroids as optimal models to examine tissue-specific responses and resistance mechanisms.
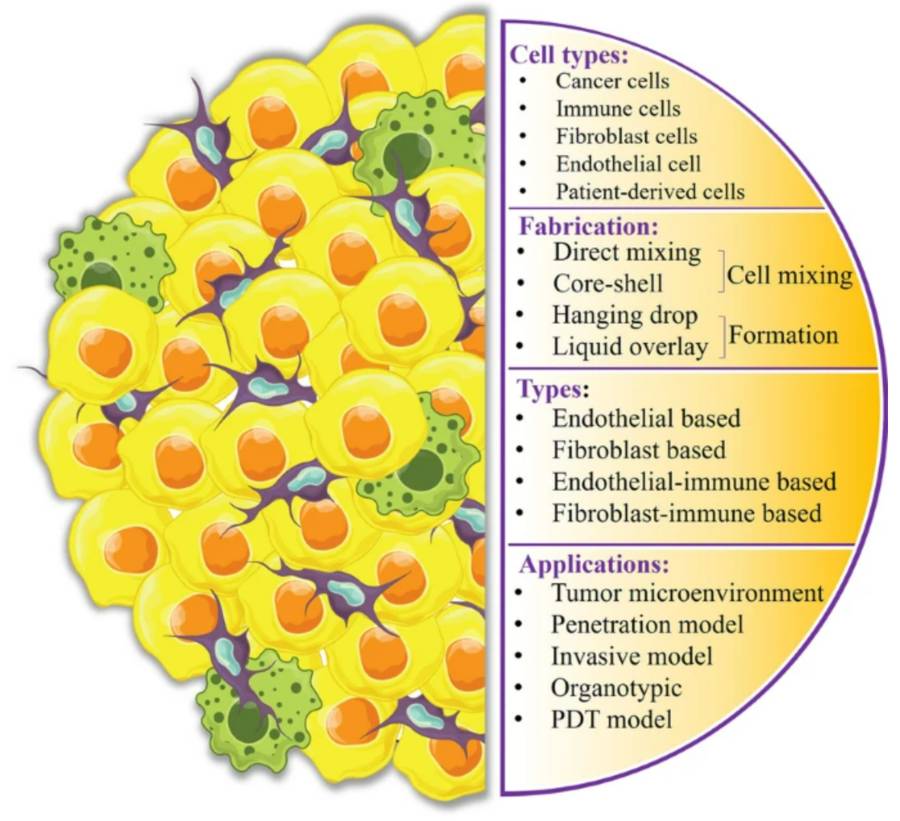 Figure 1 Heterotypic tumor spheroids Introduction.1,3
Figure 1 Heterotypic tumor spheroids Introduction.1,3
Cell Sources of 3D Spheroids
|
Cell Sources
|
Description
|
Cell Lines
|
|
Cancer Cell Lines
|
Cancer Cell Lines can form spheroids known as tumor spheroids or multicellular tumor spheroids (MCTS) which accurately represent essential characteristics of tumor biology in living organisms.
|
-
HeLa (cervical cancer)
-
A549 (lung cancer)
-
MCF-7 (breast cancer)
-
HCT116 (colon cancer)
|
|
Primary Cells
|
Primary cells obtained directly from patient tissues or animal models exhibit greater physiological relevance when compared to immortalized cell lines.
|
|
|
Induced Pluripotent Stem Cells (iPSCs) and Embryonic Stem Cells (ESCs)
|
iPSCs and ESCs produce spheroids that can develop into complex three-dimensional organoids such as brain organoids and kidney organoids while allowing researchers to explore human development and disease pathogenesis through patient or disease-specific genetic backgrounds.
|
H1, H9 (ESCs) and various patient-specific iPSC lines are widely used
|
|
Mesenchymal Stem Cells (MSCs)
|
MSCs spheroids demonstrate superior viability and secretome production along with better therapeutic outcomes than their 2D counterparts which makes them useful for regenerative medicine applications and tissue engineering as well as drug delivery studies.
|
-
Bone marrow (BM-MSCs)
-
Adipose tissue (AD-MSCs)
-
Umbilical cord (UC-MSCs)
|
Characterization of 3D Spheroids
The physiological validity and consistent performance of 3D spheroid models rely upon comprehensive characterization. The characterization process normally examines parameters that include morphology, viability, proliferation capacity, gene expression patterns, and functional properties.
Morphological Analysis
-
Size and Circularity: The routine measurement of spheroid diameter and circularity involves brightfield microscopy and specialized image analysis software. The consistency of both size and shape demonstrates effective management of the culture conditions.
-
Compaction and Density: The degree of spheroid compactness serves as a measure of the strength of cell-cell adhesion and the production of extracellular matrix material.
-
Internal Structure: Histological sectioning combined with staining methods like H&E demonstrates the internal structure of spheroids which shows necrotic cores and viable cell layers along with distinct differentiation zones especially in larger spheroids. Immunofluorescence staining enables scientists to identify particular cell types and ECM elements inside the spheroid.
Gene and Protein Expression Analysis
-
Quantitative Real-Time PCR (qPCR): Quantitative Real-Time PCR (qPCR) measures expression levels of specific genes related to cell adhesion (such as E-cadherin), tissue-specific markers, drug resistance genes or differentiation markers.
-
Western Blotting: The Western Blotting technique measures specific proteins in spheroid lysates to reveal information about signaling pathways and protein expression patterns.
-
Immunohistochemistry/Immunofluorescence: Immunohistochemistry/Immunofluorescence shows the precise location of specific proteins and cell markers in spheroid structures which provides essential insights into tissue organization and differentiation.
Functional Assays
-
Drug Sensitivity Assays: Assess how therapeutic agents produce different dose-response effects compared to 2D cultures which indicates in vivo resistance mechanisms.
-
Angiogenesis Assays: Researchers can analyze vascular network formation within spheroids that contain endothelial cells when using co-culture techniques.
-
Migration and Invasion Assays: Scientists use matrix-embedded spheroids to study cell migration and invasion that simulates metastatic behavior.
Building upon the comprehensive understanding of 3D spheroids, their effective preparation is paramount to achieving robust and reproducible models. The choice of preparation method for a cell 3D spheroid model significantly impacts spheroid size, uniformity, internal organization, and scalability.
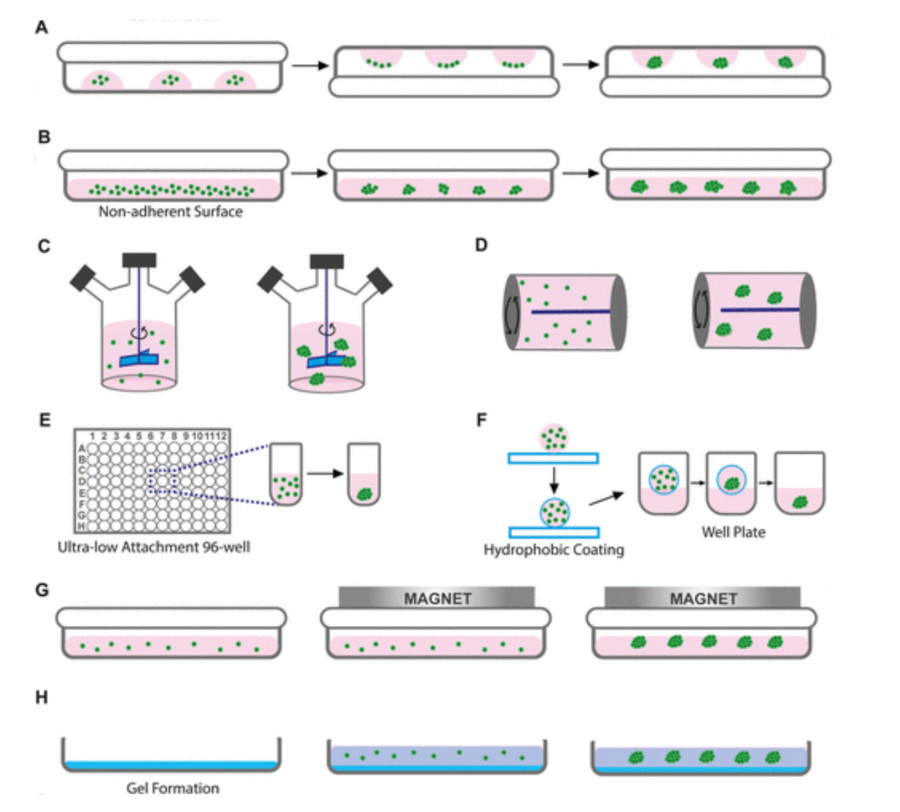 Figure 2 Conventional 3D cell culture techniques for spheroid formation.2,3
Figure 2 Conventional 3D cell culture techniques for spheroid formation.2,3
-
Ultra-Low Attachment (ULA) Plates/Surfaces - Isolate cells from a confluent 2D culture to create a single-cell suspension with a specific concentration in complete culture medium. Spheroid size and uniformity depend heavily on the initial cell density because higher densities generally produce larger spheroids.
-
Hanging Drop Method - Cells are suspended within tiny droplets of culture medium which are attached to either the underside of a tissue culture plate lid or a specialized hanging drop plate. The gravitational force causes cells to gather at the lowest part of the drop because of the concentrated cell density and lack of an adhesive surface. The air-liquid interface promotes good gas exchange.
-
Spinner Flasks and Bioreactors - Cells are cultured in a constantly stirred environment within a flask or bioreactor. The continuous agitation prevents cell attachment to the vessel walls and promotes cell-cell collisions, leading to the formation of spheroids. The stirring rate must be carefully controlled to prevent shear stress-induced cell damage.
-
Micropatterned Surfaces (e.g., Aggrewell Plates) - Cells are suspended within tiny droplets of culture medium which are attached to either the underside of a tissue culture plate lid or a specialized hanging drop plate. The gravitational force causes cells to gather at the lowest part of the drop because of the concentrated cell density and lack of an adhesive surface. The air-liquid interface promotes good gas exchange.
Advantages and Limitation of 3D Spheroids
The in vivo microenvironment is better replicated by these systems which results in more precise drug response predictions. Pre-clinical drug screenings demonstrate that 3D spheroid models can more accurately predict drug responses in living organisms than 2D cell cultures. A study showed that when using a new anti-cancer drug, the response seen in 3D tumor spheroids matched animal model responses more closely than the response in 2D cultures of the same cancer cells. These models enable researchers to study how cells interact with each other and the ECM within settings that more closely resemble physiological conditions. However, 3D spheroids also have some disadvantages. The cultivation process for these structures demands more complexity and time than traditional 2D cultures. Challenges in standardization emerge because cell seeding density, culture medium composition and culture time all influence how spheroids form and their resulting properties. Studying 3D spheroids demands more sophisticated approaches and specialized equipment than analyzing 2D cultures.
Applications of 3D Spheroids
Drug Discovery and Development: Spheroids, particularly tumor spheroids, are increasingly utilized for primary and secondary drug screens due to their ability to better mimic the drug penetration barriers and resistance mechanisms found in vivo. This leads to more accurate assessment of compound efficacy, toxicity, and adverse effects, significantly reducing the high attrition rate observed in preclinical drug development. For instance, drug compounds that appear effective in 2D cultures often fail in spheroid models or in vivo due to poor penetration or increased resistance, making spheroids crucial for early filtering.
Toxicity Evaluation: The liver-specific functions of primary hepatocytes and iPSC-derived hepatocyte spheroids endure longer than in 2D cultures. Researchers can perform more dependable evaluations of drug-induced liver injury (DILI) with primary hepatocytes or iPSC-derived hepatocyte spheroids as these systems detect acute and chronic toxicity alongside metabolic profiling and idiosyncratic reactions which conventional systems typically overlook.
Inflammatory and Autoimmune Diseases: MSC spheroids derived from UC-MSCs and BM-MSCs provide an immunomodulatory secretome which allows researchers to develop therapeutic approaches for treating inflammatory and autoimmune diseases such as rheumatoid arthritis and inflammatory bowel disease. Scientists utilize these mechanisms to explore how anti-inflammatory processes protect tissues while simultaneously using them for immunomodulatory drug testing platforms.
Frequently Asked Questions
Q: Why are 3D spheroids considered superior to 2D cell cultures for drug screening?
A: 3D spheroids create a microenvironment that more closely resembles natural tissue structure and functionality through in vivo tissue architecture replication and oxygen/nutrient gradient formation which 2D cultures cannot. The use of 3D spheroids results in precise drug penetration and metabolic profiles with accurate resistance markers which delivers predictive data for both efficacy and toxicity that aligns better with actual biological responses in living organisms.
Q: How long can 3D spheroids be maintained in culture?
A: The length of time researchers can maintain 3D spheroids in culture depends largely on the cell type used as well as the specific culture technique and media optimization approach. Immortalized cell line spheroids maintain stability for extended periods of weeks to months through routine media changes. Stem cell-derived organoids support long-term culture periods ranging from several months to more than a year which enables extensive developmental and disease progression research.
Q: What's the difference between a 3D spheroid and an organoid?
A: The terms are sometimes used interchangeably yet they represent different things. A 3D spheroid represents a broad classification for cell clusters that assemble into a spherical shape. Organoids represent advanced 3D structures from stem cells that create a self-organized system which accurately models the structural and functional properties of specific organs by differentiating into multiple cell types and resembling tissue organization found in living organisms. The entirety of organoids falls under the category of spheroids but spheroids also exist which do not qualify as organoids.
Enhance Your Research Through Creative Biolabs' 3D Spheroid
Creative Biolabs stands at the forefront of the biotechnology sector through its provision of advanced service solutions for biomedical research and drug development. Our extensive range of services supports your projects all the way from the initial idea to the final product because we understand the groundbreaking capabilities of 3D spheroid technology.
Related Services
-
Custom 3D Spheroid Generation: Produces reproducible 3D spheroids from diverse cell types, including cancer lines and patient-derived cells, customized for specific research needs.
-
3D Spheroid Based Biomarker Discovery: Services for generating and analyzing PDTS, offering a robust platform for drug response evaluation.
-
3D Spheroid Based Drug Discovery: Tumor spheroids are favored for drug screening as they effectively replicate in vivo drug penetration barriers and resistance mechanisms.
-
3D Spheroid Based Toxicity Evaluation: Spheroids serve as a more reliable platform for evaluating drug-induced toxicity, yielding better predictions of in vivo adverse effects than 2D models.
Related Products
At Creative Biolabs, we are committed to empowering your research with superior 3D cellular models, driving innovation and accelerating the translation of scientific discoveries into impactful biomedical solutions. Contact us today to learn more!
References
-
Vakhshiteh F, Bagheri Z, Soleimani M, et al. Heterotypic tumor spheroids: a platform for nanomedicine evaluation. Journal of nanobiotechnology, 2023, 21(1): 249. https://doi.org/10.1186/s12951-023-02021-y
-
Tevlek A, Kecili S, Ozcelik O S, et al. Spheroid engineering in microfluidic devices. ACS omega, 2023, 8(4): 3630-3649. https://doi.org/10.1021/acsomega.2c06052
-
Distributed under Open Access license CC BY 4.0, without modification.
Research Model
Related Sections:

 Figure 1 Heterotypic tumor spheroids Introduction.1,3
Figure 1 Heterotypic tumor spheroids Introduction.1,3
 Figure 2 Conventional 3D cell culture techniques for spheroid formation.2,3
Figure 2 Conventional 3D cell culture techniques for spheroid formation.2,3