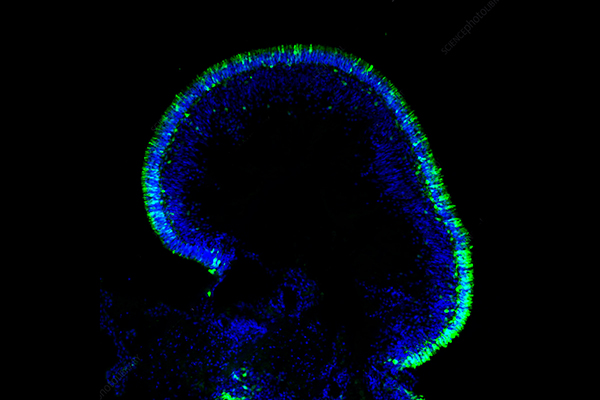
Spheroid Cell Culture Introduction
Spheroid cell culture describes the process wherein single cells naturally assemble or are directed to form rounded clusters that consist of multiple cells. Spheroids offer cells a 3D environment for interaction unlike monolayer cultures which force cells to remain on a flat surface. The dynamic environment produces a microarchitecture that mirrors the spatial organization and cellular diversity along with physiological gradients like oxygen and nutrients found in natural tissues and organs. Cell-cell adhesion along with cell density and growth factors present in the culture medium alongside nutrients govern the self-assembly process which serves as a fundamental element of embryogenesis and organogenesis. Spheroids demonstrate a natural capacity to organize and transform into functional structures which positions them as essential instruments for linking basic 2D models with intricate in vivo systems.
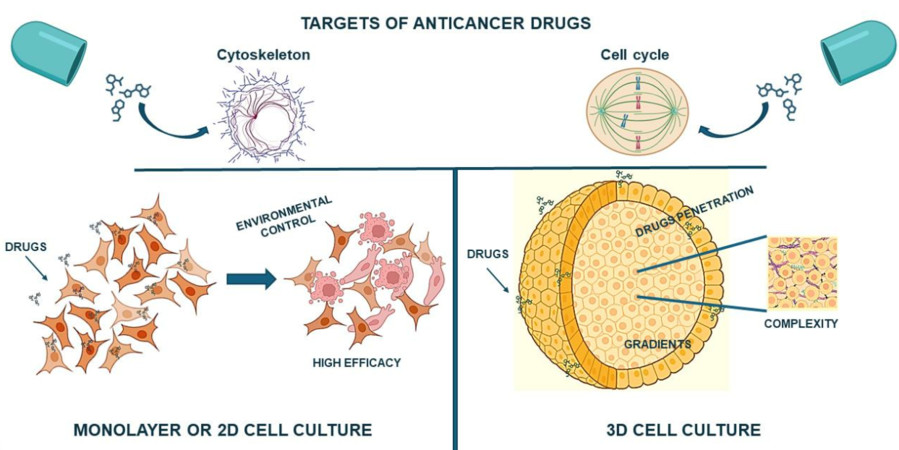 Figure 1 Two-dimensional and spheroid-based three-dimensional cell culture systems.1,3
Figure 1 Two-dimensional and spheroid-based three-dimensional cell culture systems.1,3
Spheroids in Pancreatic Cancer Cell Culture
Pancreatic cancer stands out among cancers due to its aggressive nature which makes treatment extremely challenging and its survival rate remains very low. Traditional 2D pancreatic cancer cell cultures fail to accurately represent the intricate tumor microenvironment observed in patients. Spheroid cell culture technology now serves as a powerful instrument for researchers studying pancreatic cancer biology. Pancreatic cancer cells grown in spheroid cultures organize into compact multicellular formations that mirror the natural tumor architecture found within living organisms. The pancreatic cancer spheroids comprise various cell populations such as cancer-associated fibroblasts (CAFs), immune cells, and endothelial cells which establish interactions with cancer cells. Pancreatic cancer spheroid CAFs release growth factors and cytokines which drive cancer cell proliferation and invasion while enhancing drug resistance mechanisms.
In Vitro Expansion Methods of Stem Cells in Spheroid Culture
Through spheroid culture researchers can expand different types of stem cells in vitro while achieving better stemness, viability and therapeutic outcomes than what 2D cultures offer. The three-dimensional microenvironment within spheroids facilitates essential cell-cell and cell-extracellular matrix interactions that sustain stem cell characteristics and guide differentiation. Stem cell spheroids can be produced using either scaffold-based or scaffold-free methods which offer unique benefits for large-scale production and specialized use cases.
|
Method Category
|
Principle & Advantages
|
Stem Cell Type Examples
|
|
Hanging Drop Method
|
Combines surface tension forces with gravity to bring cells together within hanging droplets. This technique delivers exact regulation of spheroid dimensions while maintaining uniformity which makes it perfect for analyzing cellular interactions. High reproducibility and suitability for high-throughput screening.
|
Mesenchymal Stem Cells (MSCs), Induced Pluripotent Stem Cells (iPSCs), Embryonic Stem Cells (ESCs)
|
|
Low/Ultra-Low Attachment (ULA) Plates
|
This method stops cells from adhering to the well surface and pushes them into suspension where they aggregate naturally because of their cell-cell adhesion properties. This method provides a straightforward solution compatible with high-throughput systems for creating spheroids that scientists commonly use. Promotes consistent formation.
|
MSCs, iPSCs, Neural Stem Cells, Cancer Stem Cells
|
|
Spinner Flask Bioreactors
|
Continuous stirring of the medium in which cells are suspended stops cell attachment while it encourages cell aggregation. The production method allows large-volume spheroid creation which suits both industrial production needs and tissue engineering applications. The manipulation of fluid dynamics allows for the optimization of spheroid dimensions.
|
MSCs, Hematopoietic Stem Cells (HSCs), Chondrocytes
|
|
Rotating Wall Vessels (RWV)
|
Creates a low-shear, microgravity-like environment by continuous rotation, keeping cells in suspension and promoting aggregation into uniform spheroids. Mimics physiological fluid dynamics, suitable for long-term culture and differentiation studies.
|
MSCs, Neural Stem Cells
|
|
Magnetic Levitation
|
Magnetic forces levitate cells mixed with magnetic nanoparticles which leads to their aggregation. Allows exact manipulation of 3D placement while supporting the creation of cell co-culture spheroids. The technique remains non-invasive and does not require labels when non-toxic nanoparticles are utilized.
|
Adipose-derived Stem Cells (ADSCs), Cancer Cells
|
|
Hydrogels (e.g., Matrigel, Alginate, Collagen)
|
A biocompatible hydrogel matrix encapsulates cells to deliver structural support while simulating the natural extracellular matrix (ECM). The system provides adjustable mechanical features and biochemical signals enabling directed cell differentiation and organization similar to natural tissue. Suitable for tissue engineering and regenerative medicine.
|
MSCs, iPSCs, Organoid cultures (e.g., gut, liver organoids from stem cells)
|
The formulation of cell culture media plays a crucial role in enabling successful formation and maintenance of spheroids. Spheroids form physiological gradients of oxygen and nutrients that span from outer regions to the core unlike 2D cultures which deliver nutrients without restriction. While standard basal media like DMEM, RPMI, or F-12K typically require serum supplementation such as FBS, scientists prefer serum-free and chemically defined media in stem cell and cancer research because these alternatives offer better reproducibility and a defined composition.
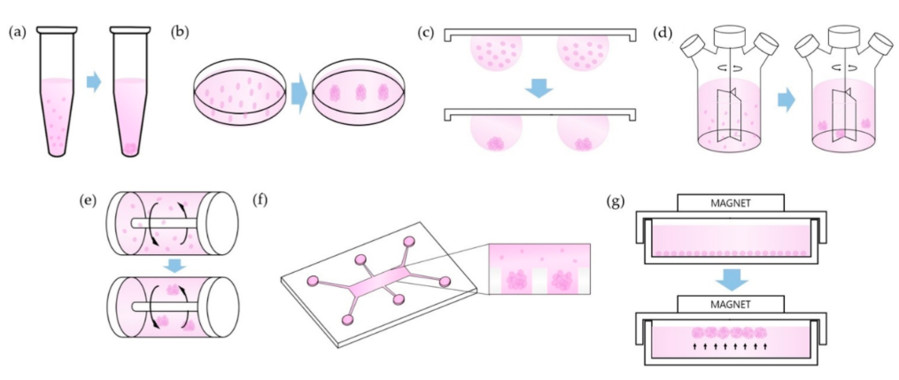 Figure 2 Cell culture in spheroid formation.2,3
Figure 2 Cell culture in spheroid formation.2,3
Key components often include:
-
Growth Factors and Supplements: These elements are crucial for supporting cellular aggregation along with their growth and specialization. Epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), insulin, transferrin and selenium serve as examples. The development of stem cells depends on niche factors including Noggin and R-Spondin as well as activators of the Wnt pathway.
-
Extracellular Matrix (ECM) Components: Some spheroids function without scaffolds while others gain structural support and better mimic natural environments through the addition of ECM components or hydrogels like Matrigel and collagen. Spheroid morphology and size as well as their functions can be affected by these components.
-
Low-Adhesion Conditions: Spheroid cultures develop self-aggregated structures through growth on ultra-low attachment plates or non-adhesive polymer-coated surfaces including poly (2-hydroxyethyl methacrylate) (polyHEMA) and agarose. Scientists select specific media and supplements based on the cell type and the targeted spheroid size to suit particular applications such as drug screening, immune response, multi-Omics analysis and regenerative medicine.
3D Spheroid Cell Culture Protocol
01 Cell Preparation and Counting
-
Digest adherent cells in the logarithmic growth phase: Discard the medium, wash twice with PBS, add trypsin until cells round up, and terminate digestion with serum-containing medium.
-
Centrifuge at 1000 rpm for 5 minutes, discard the supernatant, resuspend cells in PBS, count using a cell counting chamber, and adjust the concentration to 5×10³-5×10⁴ cells/mL (optimize based on cell type, e.g., tumor cells often use 1×10⁴ cells/well).
-
Inoculation: Add 100-200 μL of cell suspension per well in an ultra-low attachment plate, and gently shake the plate to distribute cells evenly.
-
Culture: Incubate in a CO₂ incubator, avoid frequent movement (to reduce spheroid deformation).
-
Medium replacement: Replace the medium for the first time 24 hours after culture, then replace it every 2-3 days. When replacing, gently aspirate half of the medium and add fresh medium.
03 Spheroid Formation and Observation
-
Observation frequency: Observe spheroid morphology under a microscope daily and record the formation time (micro-spheroids are usually visible within 24-72 hours).
-
Imaging and recording: Capture spheroid images using an inverted microscope and measure the diameter.
Quality Control and Optimization of Spheroid Cell Culture
|
Detection Item
|
Method
|
Standard
|
|
Spheroid diameter uniformity
|
Microscope imaging
|
Diameter coefficient of variation (CV) < 15%
|
|
Cell viability
|
Trypan blue staining or Calcein-AM/PI staining
|
Viable cell rate > 80%
|
|
Proliferation capacity
|
MTT, CCK-8, or EdU labeling
|
Proliferation curve consistent with cell cycle characteristics
|
|
3D structural integrity
|
Immunofluorescence staining (e.g., F-actin, Ki67)
|
Clear core-edge cell differentiation
|
Advantages of Spheroid Cell Culture
Spheroid cell culture provides a multitude of advantages over traditional 2D monolayer cultures, making them indispensable tools in modern biomedical research:
-
Enhanced Physiological Relevance: Spheroids replicate the natural cell environment by faithfully reproducing interactions between cells and the ECM and enabling proper signal transmission while establishing gradients of oxygen and nutrients similar to those found in tissues. This leads to more realistic cellular responses.
-
Improved Drug Screening and Discovery: Spheroids demonstrate superior predictive accuracy for drug effectiveness and toxicity because they replicate the physiological complexity of living organisms better than 2D models. Spheroids demonstrate drug penetration difficulties and the emergence of drug resistance which are typical in solid tumors.
-
More Representative Gene and Protein Expression: 3D spheroid cultured cells display gene and protein expression patterns that match those found in living tissues while maintaining native tissue architecture and functionality. Understanding disease mechanisms and therapeutic targets requires this information.
-
Disease Modeling: Spheroids help researchers build advanced disease models for cancer (tumor spheroids), neurodegenerative diseases (neural spheroids), and organ-specific pathologies which support better insights into disease progression and treatment strategies.
Frequently Asked Questions
Q: What is the main difference between 2D and 3D cell culture?
A: The main distinction between 2D and 3D cell culture methods revolves around the dimensionality through which cells expand and grow. 2D cultures limit cell growth to a flat monolayer where cells miss out on essential physiological interactions between each other and the extracellular matrix (ECM). 3D cell cultures such as spheroids enable cells to expand in every direction which results in more precise replication of actual tissue structures and heterogeneous microenvironments seen in living organisms.
Q: Why are spheroids considered better models for drug screening than 2D cultures?
A: Spheroids demonstrate improved physiological relevance due to their natural gradients of oxygen and nutrients combined with intricate cell-cell interactions. The impact of these factors on drug penetration, metabolism, and cellular response results in more precise in vivo drug efficacy and toxicity predictions while improving the understanding of drug resistance mechanisms.
Q: Can any cell type form spheroids?
A: Multiple cell types have the ability to generate spheroids but the success rate and characteristics of the produced spheroids differ. The types of cells typically used for spheroid formation include cancer cell lines and various stem cells like MSCs and iPSCs along with primary cells derived from different tissues. Each specific cell type requires the establishment of optimal conditions for proper functioning.
Conclusion
The emergence of spheroid cell culture signifies a transformation in biological modeling by overcoming the constraints of traditional 2D monolayer models. The complex tissue architecture together with cell interactions and physiological gradients of native organs are accurately replicated by these self-assembled or directed 3D cellular structures. The improved physiological relevance of these models makes them ideal for studying basic biological mechanisms as well as disease development like pancreatic cancer and stem cell behavior. Spheroids serve as essential tools in high-throughput drug screening because they provide more accurate predictions of drug efficacy and toxicity while reducing animal model use. Spheroids demonstrate transformational capabilities in biomedical research because they can be created from various methods and cell types while speeding up discovery processes and contributing to personalized medicine development.
Overview of Creative Biolabs' Spheroid Culture Services
Creative Biolabs, with its deep expertise and state-of-the-art facilities, offers comprehensive services in spheroid cell culture to empower your biomedical research. Leveraging advanced 3D culture platforms and specialized technologies, we provide tailored solutions for various applications:
Custom Services
Culture Products
3D Spheroid Model
At Creative Biolabs, we are committed to providing reliable, high-quality spheroid culture solutions that accelerate your scientific breakthroughs and contribute to the development of novel biomedical discoveries. Contact us today to learn more!
References
-
Garnique A M B, Parducci N S, de Miranda L B L, et al. Two-dimensional and spheroid-based three-dimensional cell culture systems: Implications for Drug Discovery in Cancer. Drugs and Drug Candidates, 2024, 3(2): 391-409. https://doi.org/10.3390/ddc3020024
-
Ryu N E, Lee S H, Park H. Spheroid culture system methods and applications for mesenchymal stem cells. Cells, 2019, 8(12): 1620. https://doi.org/10.3390/cells8121620
-
Distributed under Open Access license CC BY 4.0, without modification.
Research Model
Related Sections:

 Figure 1 Two-dimensional and spheroid-based three-dimensional cell culture systems.1,3
Figure 1 Two-dimensional and spheroid-based three-dimensional cell culture systems.1,3
 Figure 2 Cell culture in spheroid formation.2,3
Figure 2 Cell culture in spheroid formation.2,3