Organoid Basics
The development of organoids marks a significant breakthrough in biomedical research alongside drug development. Creative Biolabs summarized the detailed guide, which can take you step-by-step through the complete process of organoid production beginning with fundamental ideas and extending to sophisticated methods.
The table provides comprehensive details on organoid development, covering success tips, timelines, success rates, cost ranges, and key factors critical to optimizing protocols and ensuring successful outcomes.
|
Aspect
|
Details
|
Success Tips
|
|
Timeline
|
Initial formation: 3-5 day. Full maturation: 2-8 weeks
|
Patient monitoring is key
|
|
Success Rate
|
Beginners: 40-60%. Experienced: 70-90%
|
Start with simpler organoid types
|
|
Cost Range
|
$500-2000 for initial setup. $200-400 per batch
|
Optimize protocols to reduce waste
|
|
Key Factors
|
Matrix choice, growth factors, timing
|
Document everything
|
Introduction to Organoids
The development of organoids has transformed the methods researchers use to study human biology. Miniature three-dimensional cell structures known as organoids serve as advanced models that replicate real tissues for disease study and drug testing while facilitating personalized treatment development. Traditional 2D cultures develop on flat surfaces without structural complexity but organoids form complex functional units that better replicate human organs in laboratory settings.
Modern biomedical research heavily relies on organoids due to their significant advantages. Organoids have enabled revolutionary discoveries about cancer and neurodegenerative diseases along with gastrointestinal disorders and more. Because organoids can accurately mimic real tissue structures, they have become essential tools in drug discovery processes as well as personalized medical treatments and regenerative medical practices.
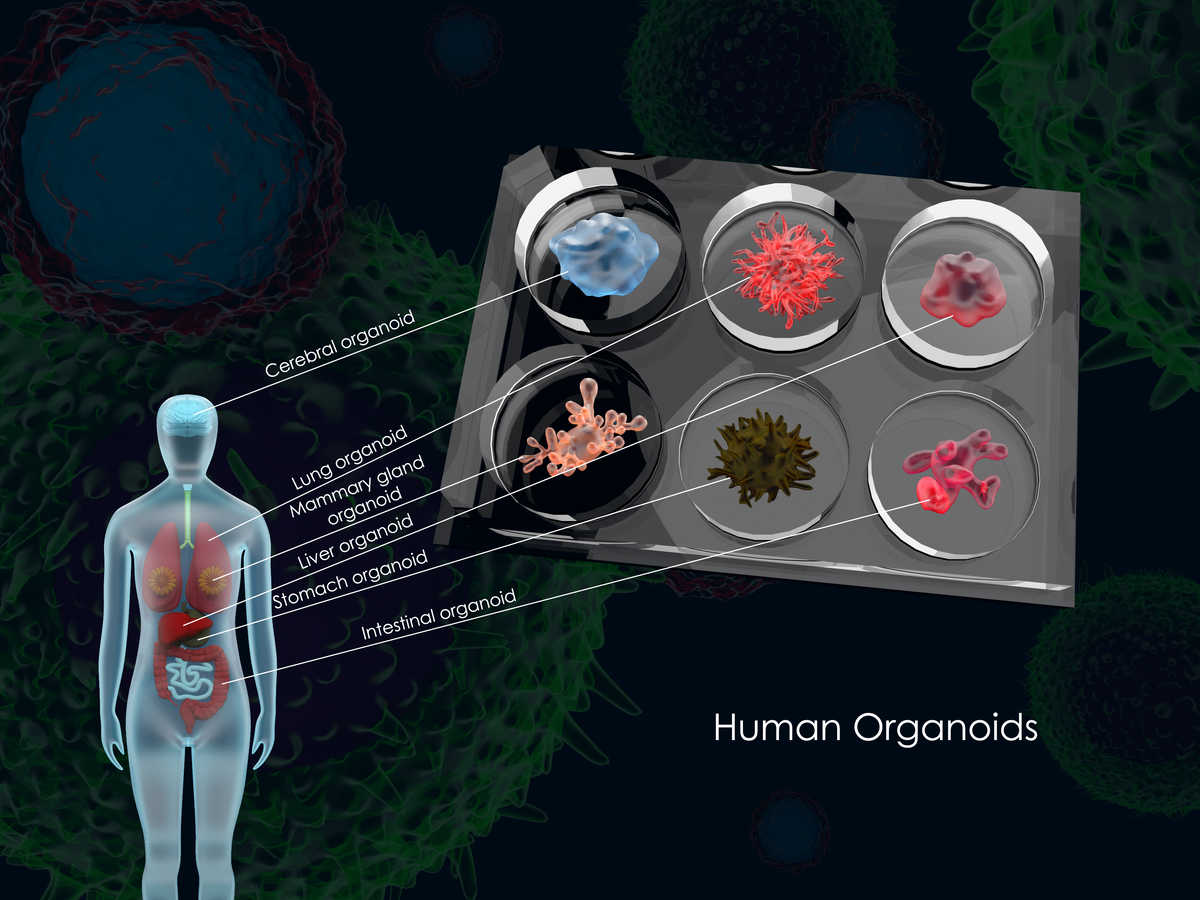 Figure 1. Common human organoids.
Figure 1. Common human organoids.
Essential Materials for Organoid Culture
To create organoids successfully, researchers need a combination of specialized cells, a supportive growth environment, and key biochemical factors. The fundamental materials required include:
-
Cell Sources: Organoids can be derived from a variety of cell types, including embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), somatic stem cells, and cancer cells. These cells serve as the foundation for organoid formation.
-
Extracellular Matrix (ECM): The ECM acts as a scaffold, allowing cells to grow and organize in three dimensions. Without a suitable ECM, organoid formation would not be possible.
-
Growth Factors and Culture Media: These biochemical supplements ensure proper cell differentiation, signaling, and survival. Specific culture media formulations help tailor the development of organoids for different tissue types.
Step-by-Step Guide to Making Organoids
Step 1: Preparing the Starting Material
Before starting, researchers must obtain and prepare their cell source. This step is crucial as it determines the quality and success of the resulting organoids.
-
Cells can be derived from fresh or cryopreserved samples.
-
Tissue dissociation involves breaking tissues down into individual cells through either mechanical (cutting, pipetting) or enzymatic (trypsin, collagenase) processes.
-
Some organoid models can also incorporate Peripheral Blood Mononuclear Cells (PBMCs) to simulate immune system interactions.
Step 2: Embedding Cells in a 3D Matrix
Once cells are isolated, they need a supportive structure to grow into organoids.
-
Cells are suspended in ECM-based gels, such as Matrigel, forming dome-like structures.
-
These structures support cellular organization, self-renewal, and differentiation, leading to the development of functional tissue-like models.
-
This step is particularly important in creating models like the 3D Tumor Organoid Model, which replicates the tumor microenvironment for cancer research.
Step 3: Culturing and Maintaining Organoids
After embedding, the culture process begins.
-
Organoids require precise environmental conditions, including stable temperature, CO₂ levels, and humidity control.
-
Culture media must be replenished every 2–3 days to ensure continued growth and function.
-
Special attention is given to different cell types, such as Primary Endothelial Cells, to optimize vascularization in organoid models.
Step 4: Passaging and Expansion
As organoids grow, they eventually require passaging to prevent overcrowding and sustain healthy expansion.
-
Passaging involves splitting mature organoids into smaller fragments, which then regrow in fresh culture conditions.
-
This can be achieved through mechanical dissection (manually breaking apart organoids) or enzymatic digestion (using dissociation enzymes).
-
Researchers carefully monitor the organoid size and density to determine the best timing for passaging.
Step 5: Long-term Storage and Cryopreservation
To ensure long-term availability, organoids can be cryopreserved.
-
Freezing is done using cryoprotective agents, such as DMSO-based solutions, which prevent ice crystal formation and cellular damage.
-
Proper freezing and thawing protocols ensure high post-thaw cell viability, particularly for models using Bone Marrow Derived Cells.
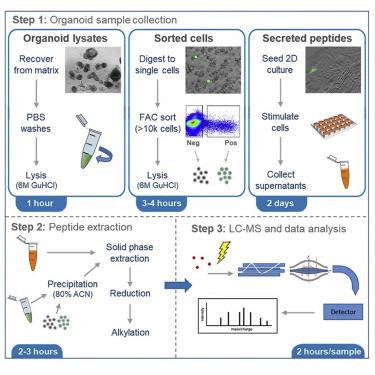 Figure. 2 Organoid Sample Preparation and Extraction for LC-MS Peptidomics.1,3
Figure. 2 Organoid Sample Preparation and Extraction for LC-MS Peptidomics.1,3
Technical difficulties and solutions of making organoid
1. Limitations of cell sources: Building organoids demands extensive quantities of primary cells or stem cells for successful development. Although researchers need numerous primary cells or stem cells to build organoids these resources remain scarce particularly when dealing with human cells. Scientists can address cell source limitations by using induced pluripotent stem cells (iPSCs) or adult stem cells because these cells can expand indefinitely in laboratory conditions and transform into any cell type via genetic and epigenetic control.
2. Challenges in mimicking the in vivo environment: The conditions used for in vitro cell culture fail to fully replicate the in vivo microenvironment which includes the immune system surrounding cells and this discrepancy can lead to variations in cell growth and differentiation and create problems during organoid development. Developing new culture techniques and materials such as microfluidic chips and biomaterials offers potential solutions to replicate cells' physical and chemical in vivo environment. Simulating the in vivo environment becomes more accurate when exogenous cells like immune cells are incorporated into tumor-like organs to mimic tumor microenvironments.
3. Challenges of complexity and scale of organoids: The existing organoids have reached neither the size nor the structural complexity of natural organs which restricts their practical uses. Researchers propose using 3D printing and bioreactor technologies to create organoids that are both larger in size and greater in complexity.
4. Challenges in functional validation of organoids: Scientists typically assess organoid function through animal model testing but these tests often fail to replicate human physiological conditions accurately. Developing fresh in vitro validation approaches like drug screening and toxicity testing through microfluidic chip systems represents one potential solution.
5. Challenges of time and cost for organoid culture: The cultivation of organoids needs extended incubation durations and substantial financial investment. Researchers can improve cell growth speed and efficiency while cutting costs by fine-tuning culture methods and conditions for specific tissues or organs.
Optimizing Organoid Growth and Quality
To produce high-quality organoids, researchers implement several optimization strategies:
-
Pre-wetting plastics with basal media to prevent unwanted cell adhesion.
-
Selecting the right combination of growth factors depending on the tissue model (Purified Immune Cell Populations).
-
Ensuring strict sterile conditions to avoid microbial contamination.
Applications of Organoids in Research and Medicine
Organoids have enabled advancements in:
-
Drug discovery and testing, allowing researchers to screen potential treatments using patient-derived models. The Precision Cut Tissue Slicing Model is an example of such applications.
-
Personalized medicine, where doctors test drug responses on patient-specific organoids before prescribing treatments.
-
Disease modeling, with organoids being used to study conditions like cancer, genetic disorders, and neurodegenerative diseases.
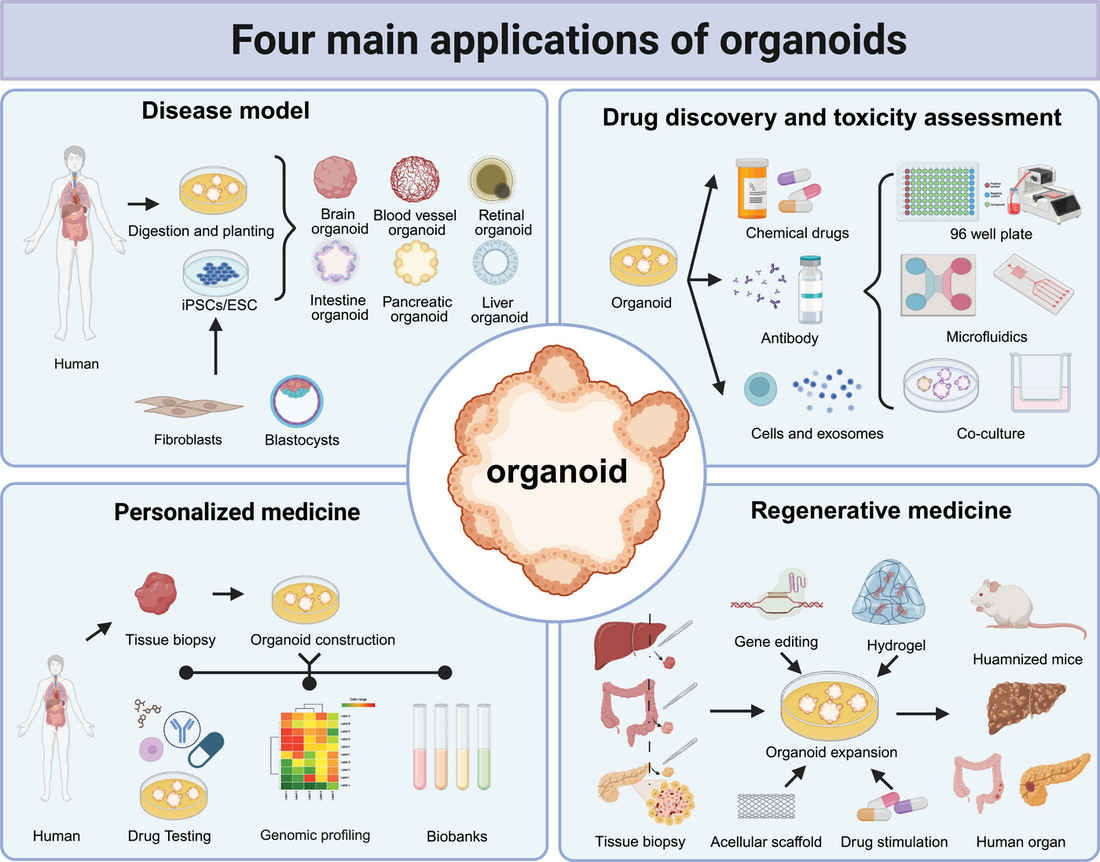 Figure 3. Four main applications of organoids.2,3
Figure 3. Four main applications of organoids.2,3
Looking to the Future
The organoid field is evolving rapidly. Here's what's on the horizon:
-
Vascularized Organoids
-
Integration of functional blood vessels
-
Improved nutrient delivery
-
Enhanced tissue maturation
-
Applications in transplantation research
-
Multi-Organ Systems
-
Organ-organ interaction studies
-
Metabolic pathway analysis
-
Systemic disease modeling
-
Drug metabolism research
-
Brain Organoid Advancement
-
Neural circuit formation
-
Consciousness studies
-
Mental disorder modeling
-
Developmental neurobiology
Frequently Asked Questions (FAQs)
Q: How should we estimate the time required for each process of organoid culture? For example, how to determine the correct time point to observe the formation of organoids, and even the subsequent medium change and passaging?
A: If you choose to use adult stem cells to build organoids, it usually takes 1-2 weeks to build successfully. If you use ESC/iPSC to build organoids, it often takes longer. For specific culture methods, time required, medium change and passaging frequency at each stage, please refer to relevant literature information.
Q: Can organoids be genetically modified?
A: Scientists working in research use CRISPR technology together with viral vector methods to edit organoid genes.
Q: Are there any standards for successful organoid establishment?
A: After constructing the organoid model, some methods are needed to compare the similarity between the constructed organoid and the physiological tissue. The successfully cultured organoids have clear and transparent edges under an optical microscope, and their morphology is spherical or vesicular depending on the corresponding tissue (small intestinal organoids show crypt-villus-like structures).
Organoids generally include the main cell types of the corresponding tissue. Organoids can be identified from multiple dimensions such as morphology and molecular genetics through methods such as HE staining, immunohistochemistry, immunofluorescence and gene sequencing. If it is a tumor organoid, in addition to containing tumor biomarkers, it should also have the heterotypic characteristics of tumor cells (pathological identification). Organoids can be stably passaged, frozen and revived.
Expert Tips and Tricks
After years of hands-on experience, here are detailed insights that can make or break your organoid research:
Protocol Optimization
-
Temperature Management
-
Maintain consistent incubator temperature (±0.2°C)
-
Pre-warm all media and reagents
-
Use temperature-controlled centrifugation
-
Monitor room temperature fluctuations
-
Timing Considerations
-
Create detailed schedules for media changes
-
Plan experiments around critical time points
-
Document all procedural delays
-
Build in buffer time for unexpected issues
-
Quality Control Measures
-
Implement regular sterility checks
-
Monitor growth patterns daily
-
Record morphological changes
-
Maintain detailed logs
Conclusion: The Future of Organoid Research
The expanding domain of organoid technology creates transformative advancements throughout medicine, drug development research, and disease investigation. Creative Biolabs specializes in advancing organoid research through our leading-edge 3D Biology solutions which feature custom cell culture services and ECM materials along with expert guidance.
Cutting-Edge 3D Services of Creative Biolabs
High-Quality Primary Cells & Ex Vivo Models
References
-
Emily L. Miedzybrodzka, Rachel E. Foreman, et al. Organoid Sample Preparation and Extraction for LC-MS Peptidomics. STAR Protocols. 2020, 1, 100164. https://doi.org/10.1016/j.xpro.2020.100164.
-
Yao Q, Cheng S, Pan Q, et al. Organoids: development and applications in disease models, drug discovery, precision medicine, and regenerative medicine. MedComm. 2024; 5:e735. https://doi.org/10.1002/mco2.735
-
Distributed under Open Access license CC BY 4.0, without modification
Research Model
Related Sections:

 Figure 1. Common human organoids.
Figure 1. Common human organoids.
 Figure. 2 Organoid Sample Preparation and Extraction for LC-MS Peptidomics.1,3
Figure. 2 Organoid Sample Preparation and Extraction for LC-MS Peptidomics.1,3
 Figure 3. Four main applications of organoids.2,3
Figure 3. Four main applications of organoids.2,3