What is the 3D Spheroid Invasion Assay?
Researchers utilize the 3D spheroid invasion assay as a sophisticated laboratory technique to measure cell invasion ability in an authentic 3D cellular environment. 3D spheroids deliver a precise emulation of solid tumors and tissue micro-regions which traditional 2D cell culture systems cannot achieve since they fail to replicate essential features of in vivo tumor structure and cell movement.
Principles of 3D Spheroid Invasion Assay
The assay's fundamental process includes producing multicellular spheroids which are cell clusters that form into dense spherical shapes on their own. After formation researchers place the spheroids into a three-dimensional matrix usually made from extracellular matrix materials including Matrigel or collagen. The invasive characteristics of the cells are assessed through time-lapse monitoring of their expansion and movement from the dense spheroid into the adjacent matrix structure. Non-invasive cells maintain their compact spherical structure with clear boundaries, whereas invasive cells and endothelial cells show characteristic spread and movement through the extracellular matrix. The dynamic process considers important pathophysiological factors such as hypoxia and nutrient deprivation inside the spheroid that significantly affect gene expression and encourage migratory and invasive behaviors which resemble in vivo conditions.
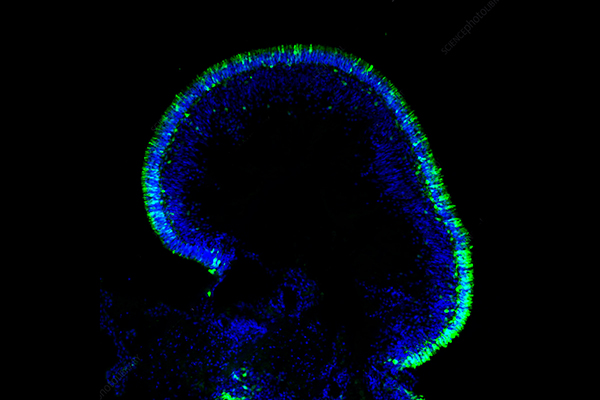
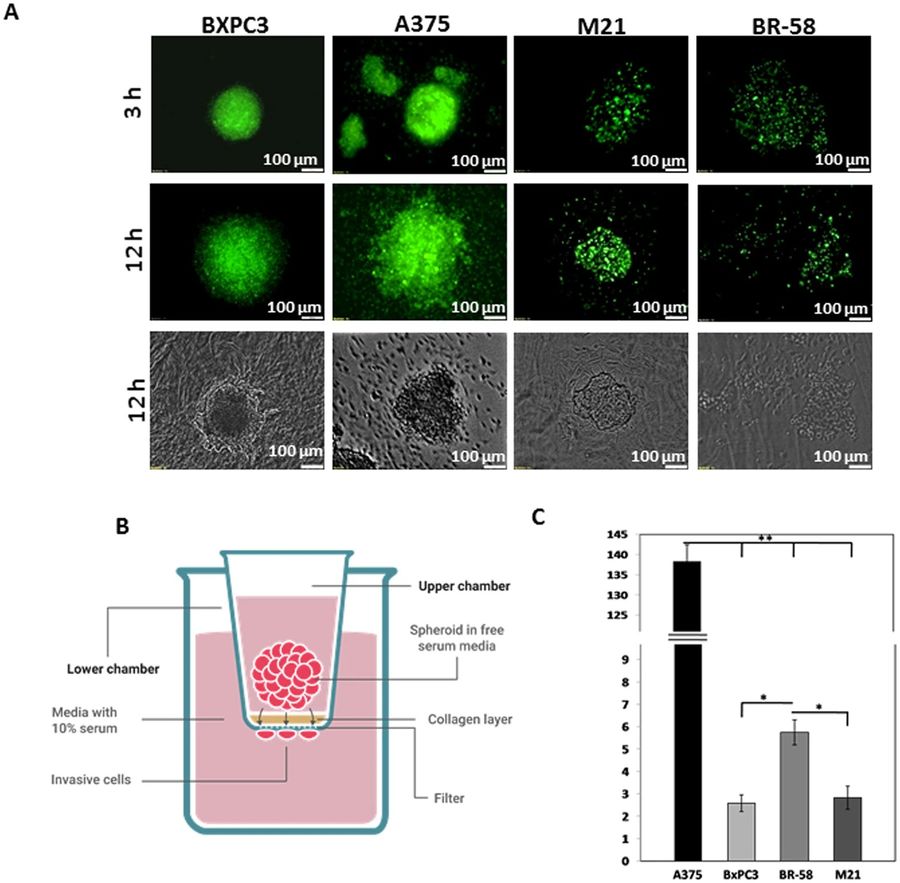 Figure 1 Spatial spheroid invasion assays.1
Figure 1 Spatial spheroid invasion assays.1
Microcarrier-Based Spheroid 3D Invasion Assay
Microcarrier-based systems provide distinctive benefits over traditional hanging drop and ultra-low attachment plate methods for generating spheroids especially in high-throughput applications.
Methodology of Microcarrier-Based Spheroid 3D Invasion Assay
The microcarrier-based technique involves seeding cells onto specialized microscopic beads which serve as scaffolds to help form spheroids. The surface chemistries of microcarriers are specifically engineered to support cell aggregation and attachment which results in the creation of uniform spheroids. After spheroids develop on the microcarriers researchers can effortlessly handle and move them into an invasion matrix. The system produces many uniform spheroids that enable consistent and reliable outcomes for drug screening, multi-omics analysis, immune response analysis, target analysis and validation, and extensive research studies. Microcarriers provide a controlled environment that enables both desired spheroid size attainment and improved homogeneity which other methods struggle to achieve.
Advantages of Microcarrier-Based Spheroid 3D Invasion Assay
Microcarrier-based spheroid invasion assays offer several benefits:
-
Scalability and High-Throughput: The combination of simple handling and consistent spheroid production makes them ideal for automated processes and high-throughput screening (HTS).
-
Reproducibility: The controlled conditions of microcarriers generate uniform spheroid dimensions and forms which minimizes variability across experimental replicates.
-
Enhanced Physiologically Relevance: Microcarriers deliver a precise framework that directs spheroid formation to resemble the early tumor nodule development stages found in living organisms.
-
Ease of Handling: Cultivating spheroids on microcarriers enables straightforward transfer and culture which streamlines subsequent analysis processes such as embedding in invasion matrices and imaging.
Breast Cancer Spheroid Invasion Collagen Assay
The dissemination of cancer cells from the primary breast tumor to remote body areas is the primary reason for death in breast cancer patients. Researchers must study this process in vitro to gain insights into disease progression and create successful therapies. The breast cancer spheroid invasion collagen assay provides a reliable model for studying tumor invasion.
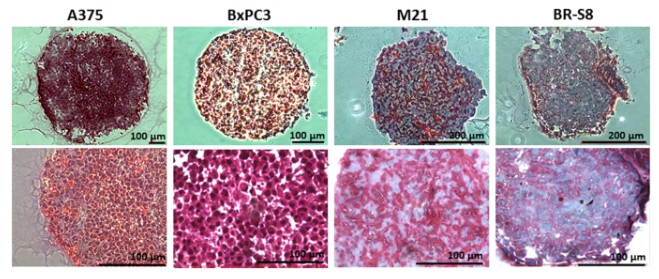 Figure 2 Histological staining for Collagen level in spheroid sections.1
Figure 2 Histological staining for Collagen level in spheroid sections.1
Relevance: The process of tumor invasion involves an intricate relationship between cancer cells and their adjacent stromal environment which necessitates the movement through a compact network of collagen type I fibers that form the main structure of the extracellular matrix in various tissues such as breast tissue. The use of breast cancer spheroids within collagen type I gel enables scientists to replicate the physiological barrier which cancer cells encounter during invasive processes.
Mechanism: Researchers grow breast cancer cell lines including the aggressive triple-negative MDA-MB-231 and the more docile luminal MCF-7 to form 3D spheroids. A collagen hydrogel serves as the embedding material for these spheroids. Researchers track both the expansion and structural changes of invading cells through live-cell imaging techniques. The extent to which cells invade the collagen matrix along with changes in their structure and F-actin arrangement can be quantified. This assay is particularly valuable for:
-
Investigating Cell-ECM Interactions: The system enables researchers to analyze breast cancer cell interactions and degradation activities within the collagen matrix which is essential for metastasis progression.
-
Evaluating Anti-Invasive Agents: Scientists frequently apply this assay to preclinical evaluation of small-molecule inhibitors and biologic compounds that aim to block breast cancer invasion pathways. Scientists can examine compounds that block matrix metalloproteinases (MMPs) to determine if they prevent spheroid invasion into collagen structures.
-
Understanding Cancer Cell Plasticity: The assay demonstrates how breast cancer cells modify their phenotype according to both mechanical and biochemical signals from their collagen-rich surroundings.
Evaluation of Consistency in Spheroid Invasion Assays
The utility of any in vitro assay hinges on its consistency and reproducibility. Achieving high consistency in spheroid invasion assays is paramount for reliable data interpretation and meaningful comparisons across experiments. To enhance consistency, researchers employ several strategies:
-
Standardized Spheroid Formation: Researchers achieve uniform spheroid size and shape through the use of ultra-low attachment plates combined with optimized cell seeding densities or microfluidic platforms.
-
Automated Image Analysis: Using automated and semi-automated image analysis software with objective segmentation algorithms reduces operator bias while enabling high-throughput analysis of invasion metrics including invaded area and mean invasion distance. The advanced algorithms can measure nuclear pixel distances relative to the starting spheroid boundary to deliver exact integrative measures that depend on direction during invasion tracking.
-
Defined ECM Protocols: Following established ECM preparation protocols for thawing, dilution, and polymerization maintains uniform matrix properties.
-
Real-Time Monitoring: Live-cell imaging systems enable researchers to track invasion processes over time by delivering kinetic data and allowing observation of cellular behavior dynamics. The ongoing data feed serves as an early warning system for detecting and addressing problems with spheroid health or experimental conditions.
Spheroid Invasion Assay for T Cell Cytotoxicity
3D spheroid models serve as essential tools for studying immune cell activity including T cell tumor-killing capabilities and provide a more accurate testing environment than traditional 2D co-culture models. Scientists produce tumor spheroids which they then expose to T cells in co-culture experiments using different effector-to-target ratios. Researchers track T cell movement into tumor spheroids and measure tumor size decrease and viability loss from T cell killing. Researchers can utilize fluorescent dyes to mark tumor cells and T cells along with dead cells which enables scientists to assess cytotoxic effects quantitatively through live-cell imaging techniques in real time.
Comparative Analysis of 3D Spheroid Invasion Assays and Traditional 2D Model Assays
|
Feature
|
Traditional 2D Models (e.g., Transwell, Scratch Assay)
|
3D Spheroid Invasion Assays
|
|
Simplicity & Setup
|
Easy to set up; low equipment requirements.
|
Technically demanding; requires optimization for spheroid formation and embedding.
|
|
Cost
|
Lower cost for reagents and consumables.
|
Higher cost due to specialized reagents, equipment, and consumables.
|
|
Cell Morphology & Polarity
|
Cells lose natural morphology and polarity; flatten on rigid surfaces.
|
Cells maintain natural 3D morphology and polarity; self-assemble.
|
|
Cell-Cell/ECM Interactions
|
Limited or altered interactions.
|
Promotes authentic cell-cell and cell-ECM interactions, closer to in vivo.
|
|
Environmental Gradients
|
Uniform access to nutrients, oxygen, signals; not representative.
|
Forms physiological gradients (oxygen, nutrients, pH); simulates solid tumors.
|
|
Gene/Protein Expression
|
Expression profiles differ from in vivo.
|
Expression profiles are closer to in vivo tissues.
|
|
Cell Proliferation
|
Faster proliferation rates.
|
Variable proliferation zones (proliferative, quiescent, hypoxic); generally slower overall.
|
|
Drug Penetration
|
Uniform and unrestricted drug access.
|
Drug penetration limited by 3D structure and gradients, more realistic.
|
|
In Vivo Predictivity
|
Poor predictivity; results difficult to translate to in vivo.
|
Enhanced predictivity; more accurate data for drug efficacy and toxicity.
|
|
Model Complexity
|
Limited ability to simulate complex biological phenomena.
|
More suitable for simulating complex interactions (collective migration, immune cell infiltration).
|
|
Timeframe
|
Faster, typically shorter assay durations.
|
Longer assay durations (days to weeks) for spheroid formation and invasion.
|
|
High-Throughput Potential
|
High, suitable for initial large-scale screening.
|
High potential with automated systems and microcarrier methods, but more complex.
|
|
Applications
|
Initial compound screening, basic mechanistic studies.
|
Target validation, lead optimization, cell assay, personalized medicine, complex disease modeling (e.g., metastasis, immunotherapy).
|
Frequently Asked Questions
Q: Why choose a 3D spheroid invasion assay over a 2D invasion assay?
A: Invasion assays using 3D spheroids provide a much higher level of physiological relevance. The 3D spheroid invasion assays provide an accurate representation of the in vivo tumor microenvironment through cell-cell contacts and cell-extracellular matrix interactions along with nutrient and oxygen gradient formation. Drug efficacy studies and complex biological process research benefit from more precise and predictive data derived from 3D assays than from basic 2D models.
Q: What types of cells can be used to form spheroids for invasion assays?
A: Researchers can choose from multiple cell types to form spheroids for invasion assays including various cancer cell lines like breast, prostate and lung alongside glioblastoma, primary tumor cells, endothelial cells, fibroblasts and immune cells. The selection of a cell line for research depends on both the research question and the particular invasion mechanism under investigation.
Q: What extracellular matrices are commonly used in spheroid invasion assays?
A: The commonly utilized ECMs in assays are Matrigel (which is derived from basement membranes), collagen Type I (used often to replicate stromal invasion), fibrin and synthetic hydrogels. The selected extracellular matrix depends on the physiological context because different matrices vary in stiffness, pore size, and biochemical characteristics.
Conclusion
The 3D spheroid invasion assay serves as a powerful laboratory technique that accurately replicates cell invasion processes in living organisms under physiological conditions. This assay requires the creation of multicellular spheroids from cancer cells which are then embedded into a three-dimensional extracellular matrix. Scientists measure how cells move outward from the spheroid to obtain essential information about their invasive nature. This assay proves essential for cancer metastasis research while providing superior predictive information for anti-invasive drug discovery and immune cell-tumor interaction studies compared to traditional 2D models. Its enhanced physiological relevance together with quantifiable results establish it as a fundamental method for preclinical studies despite its complexity and time-consuming nature.
Discover What Creative Biolabs Can Offer You
Creative Biolabs stands at the forefront of in vitro assay development innovation with a special focus on 3D cell culture systems. Our specialized scientific knowledge along with leading-edge platforms enables us to deliver complete 3D spheroid invasion assay services customized to support your research and drug development objectives.
Custom Services
Culture Products
3D Spheroid Model
Partnering with Creative Biolabs provides access to dependable and accurate 3D invasion assays that speed up biomedical research while helping validate therapeutic targets and enhance drug development processes. Contact us today to learn more!
Reference
-
Shoval H, Karsch-Bluman A, Brill-Karniely Y, et al. Tumor cells and their crosstalk with endothelial cells in 3D spheroids. Scientific Reports, 2017, 7(1): 10428. https://doi.org/10.1038/s41598-017-10699-y. Distributed under Open Access license CC BY 4.0, without modification.
Research Model
Related Sections:

 Figure 1 Spatial spheroid invasion assays.1
Figure 1 Spatial spheroid invasion assays.1
 Figure 2 Histological staining for Collagen level in spheroid sections.1
Figure 2 Histological staining for Collagen level in spheroid sections.1