Patient-Derived Xenografts (PDX) Model Introduction
PDX models are a highly useful preclinical tool for cancer research. Unlike cell lines, which lose the original genetic and histological features of the initial tumor with prolonged in vitro culture, the PDX model implants the patient tumor tissues directly into immunocompromised mice to grow and expand in the in vivo environment mimicking human physiology. Accordingly, the preclinical model retains the most significant aspects of the original primary tumor including tissue architecture, gene mutations, gene expression profiles and cellular heterogeneity. The fidelity of the original patient tumor to the PDX model is an invaluable asset for the prediction of clinical responses to anti-cancer treatments, drug development, biomarker discovery, identifying novel therapeutic targets, and developing personalized treatment strategies.
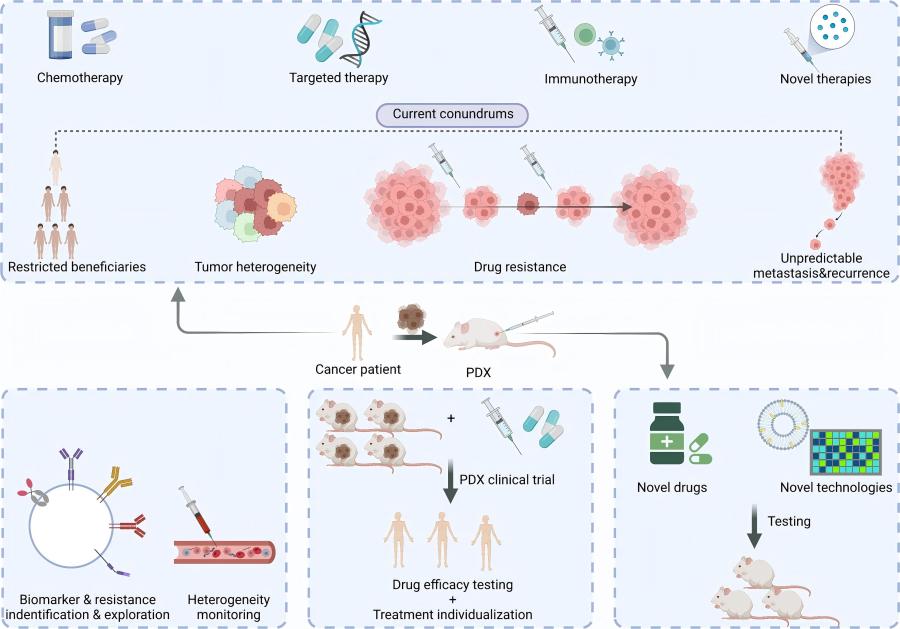 Figure 1 PDX in the new era of cancer treatment.1,4
Figure 1 PDX in the new era of cancer treatment.1,4
What is PDX Mouse Models?
The principle of the PDX model is immunodeficiency in mice that carry tumors derived from human specimens because nude mice or SCID mice (DNA double strand break repair-deficient and T and B lymphocyte-deficient) used as hosts have no resistance to tumors composed of human material. These mice will accept human tumors without immune rejection and can be used for and measuring tumorigenesis and treatment efficacy free of interference. The PDX model has greater utility than in vitro cell line models and genetically engineered mouse models (GEMM) for the following reasons:
-
PDX models preserve the original tumor's cellular diversity and clonal structure, essential for studying drug resistance and tumor progression.
-
Murine stromal components replace human ones over time, but early PDX passages reflect critical tumor-microenvironment interactions affecting drug response.
-
PDX model responses closely align with patient outcomes, supporting their relevance in clinical trials.
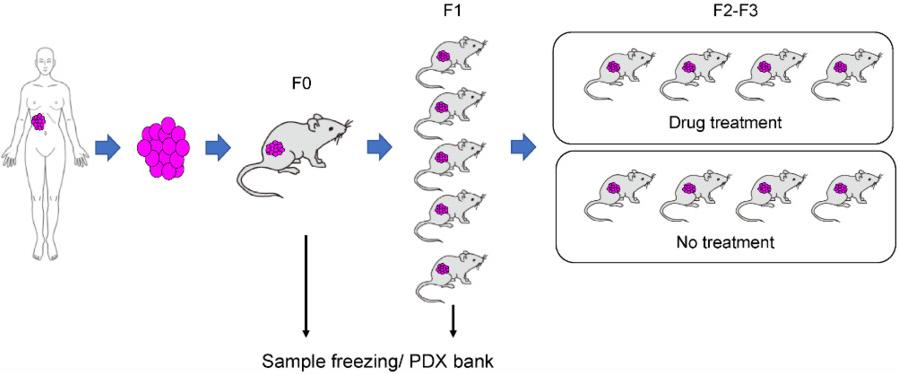 Figure 2 Applications of PDX mouse models.2,4
Figure 2 Applications of PDX mouse models.2,4
PDX Tumor Model Types
PDX tumor models encompass a wide range of cancer types, making them versatile tools in oncology:
-
Solid Tumor Models: PDX models have been generated for most solid tumors, including breast cancer, lung cancer, colorectal cancer, ovarian cancer, pancreatic cancer, gastric cancer, glioblastoma, and melanoma. These PDX models are valuable for evaluating new targeted therapies, immunotherapies, and combination regimens.
-
Hematological Malignancy Models: PDX models for leukemias and lymphomas are more challenging to generate because of the highly disseminated nature of the diseases, however, by injecting patient-derived cells into the tail vein or the femoral vein, PDX models of hematological malignancies can be established. This method can mimic systemic disease development.
-
Metastatic Models: Advanced PDX models have also been established to study metastatic disease. These models can be generated through orthotopic implantation or by directly injecting tumor cells into the circulation, enabling the study of metastatic cascade and evaluating anti-metastatic drugs.
-
Drug Resistance Models: PDX models can be generated from the tumor which has relapsed or developed drug resistance to the standard treatment. Researchers can study the mechanisms of drug resistance and find solutions to overcome it.
Comparison of Patient-Derived Models: PDX, PDO, and PDC
|
Feature
|
Patient-Derived Xenograft (PDX)
|
Patient-Derived Organoid (PDO)
|
Patient-Derived Cell (PDC)
|
|
Description
|
Tumor tissue from a patient directly implanted into an immunodeficient mouse.
|
3D cell cultures grown in vitro from patient tumor cells, forming mini-organs that mimic the original tumor's architecture and function.
|
2D (or sometimes 3D) cell cultures derived directly from patient tumor cells, typically grown as monolayers.
|
|
Environment
|
In vivo (within a living mouse), mimicking the tumor microenvironment, vasculature, and stromal interactions.
|
In vitro (in a lab dish) within a supportive 3D matrix (e.g., Matrigel), allowing for self-organization of cells into complex structures.
|
In vitro (in a lab dish), typically as adherent cells on a flat surface, with limited mimicry of the original tumor microenvironment.
|
|
Development Time
|
Long (several months for establishment and expansion).
|
Relatively short (weeks to a few months).
|
Short (weeks).
|
|
Throughput
|
Low-Medium (limited by the number of animals that can be managed).
|
High (suitable for high-throughput drug screening).
|
High (most amenable to high-throughput drug screening).
|
|
Predictive Value
|
Generally considered the most clinically predictive for drug efficacy, as it models the in vivo environment.
|
Good; increasingly recognized for its ability to predict patient drug responses, often correlating well with PDX data.
|
Variable; generally better than established cell lines but may not fully capture complex drug responses due to lack of microenvironment.
|
|
Applications
|
Preclinical drug efficacy testing, biomarker discovery, personalized medicine (patient avatars), studying tumor evolution.
|
Drug screening, personalized medicine, disease modeling, studying tumor heterogeneity, CRISPR/gene editing studies.
|
Initial drug screening, molecular profiling, studying basic cell biology, toxicology studies.
|
PDX Model Generation Protocol
Creating a solid and dependable PDX model needs detailed attention and careful adherence to certain procedures. Although steps can be somewhat varied depending on the kind of tumor and the aims of the study, the overall protocol is:
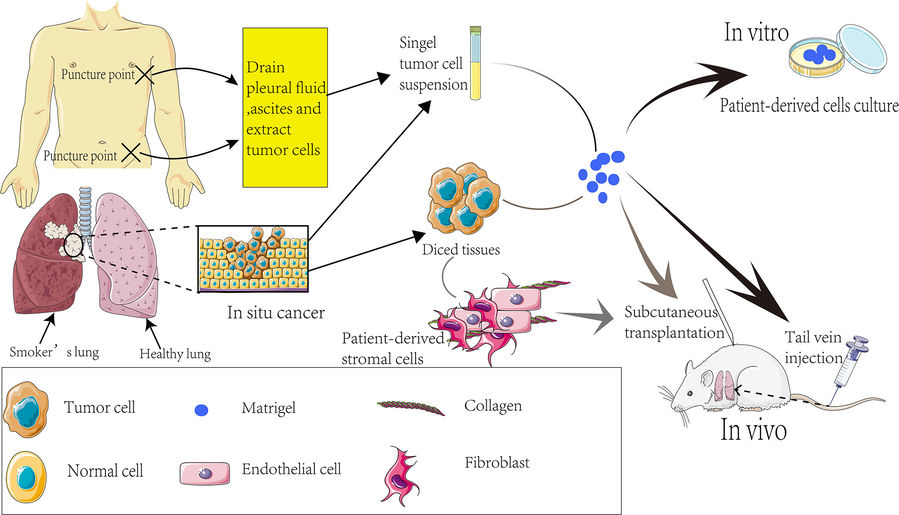 Figure 3 Establishment process of PDX models.3,4
Figure 3 Establishment process of PDX models.3,4
-
Patient tissue processing: Within 30 minutes of resection, tumors are minced into 1-3 mm³ fragments in chilled preservation medium.
-
Processing of patient tissues: Tumors are surgically excised and, within 30 min of removal, cut into pieces of 1-3 mm³ in chilled preservation medium.
-
Multi-site implantation: Fragments were implanted subcutaneously (flank) or orthotopically (liver, pancreas) by means of trocar needles.
-
Monitoring for engraftment: Tumor growth was monitored by measuring dimensions with a caliper; successful takes could usually be observed between 2 and 8 weeks.
Challenges of PDX Models
The PDX model is widely used in cancer research due to its faithful representation of human tumors, but its ability to mimic human biology is still subject to certain technical limitations. The use of immune deficient host models is particularly limited when evaluating immune based therapies or measuring the immune microenvironment of tumors. Another technical challenge of building PDX models is that they are lengthy, expensive, and labor-intensive. The low implantation rate and replacement of mouse stromal cells in continuous passage may lead to a decrease in the authenticity of tumors. In order to fully utilize the predictive ability of PDX models in oncology, these technical challenges must be addressed.
Frequently Asked Questions (FAQs)
Q: How do PDX models compare to cancer cell lines?
A: PDX models more closely recapitulate the genetic, histological and molecular features, and the heterogeneity of the patient tumor that is often not retained in conventional 2D cell lines. Furthermore, they develop in vivo in a more physiologic niche and have better predictability to patient response.
Q: How long does it take to create a PDX model?
A: Engraftment is incredibly variable between cancer types and individual tumors and can be observed take between weeks (4-6) to months. Later paragraphs usually strengthen up and grow faster.
Q: Do PDX models maintain the human tumor microenvironment?
A: Initially, the PDX model retains some human stromal components. However, over successive passages, the human stroma is gradually replaced by murine stroma. Despite this, the tumor cells themselves retain their human origin and key tumor-specific characteristics. For studies requiring human immune cells, humanized PDX models are available.
Elevate Your Research with Creative Biolabs' Advanced PDX Models
Creative Biolabs is a global leading service provider, who is specialized in clinical and preclinical studies for Patient-Derived Xenograft (PDX) models and patient-derived organoids. Scientific Excellence and Quality Control Our dedication to scientific quality and stringent quality control make our models highly reliable and clinically relevant.
Creative Biolabs is dedicated to leveraging cutting-edge biomedical technologies, including PDX models, to accelerate discoveries that translate into effective therapies. Contact us today to learn more!
References
-
Liu Y, Wu W, Cai C, et al. Patient-derived xenograft models in cancer therapy: technologies and applications. Signal Transduction and targeted therapy, 2023, 8(1): 160. https://doi.org/10.1038/s41392-023-01419-2
-
Goto T. Patient-derived tumor xenograft models: toward the establishment of precision cancer medicine. Journal of personalized medicine, 2020, 10(3): 64. https://doi.org/10.3390/jpm10030064
-
Liu W, Cui Y, Zheng X, et al. Application status and future prospects of the PDX model in lung cancer. Frontiers in Oncology, 2023, 13: 1098581. https://doi.org/10.3389/fonc.2023.1098581
-
Distributed under Open Access license CC BY 4.0, without modification.
Research Model
Related Sections:

 Figure 1 PDX in the new era of cancer treatment.1,4
Figure 1 PDX in the new era of cancer treatment.1,4
 Figure 2 Applications of PDX mouse models.2,4
Figure 2 Applications of PDX mouse models.2,4
 Figure 3 Establishment process of PDX models.3,4
Figure 3 Establishment process of PDX models.3,4