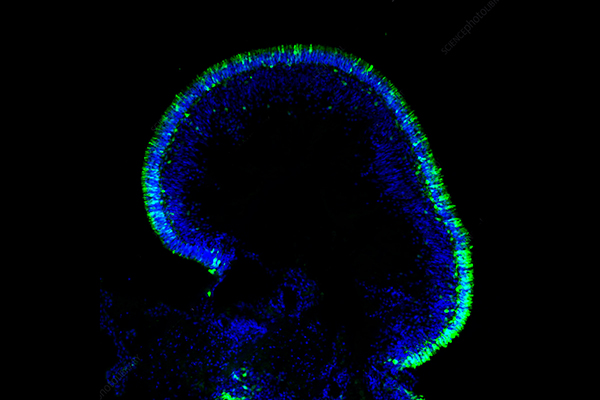
Organoids Definition
An organoid is a group of cells that are differentiated from stem or progenitor cells in an in vitro three-dimensional (3D) culture model. It has a structure and some functions similar to real organs. By imitating the development process of organs in the body, organoids can somewhat replicate the physiological activities and structural features of natural tissues or organs.
Organoids Characteristics
-
Diversity of cell types: organoids must contain more than one cell type that is identical to the source organ.
-
Functional similarity: organoids should exhibit some specific functions of the source organ.
-
Spatial similarity: organoids should be organized in a cellular manner similar to that of the source organ.
-
Self-renewal and assembly ability: the organoid is capable of self-assembly and differentiation through cell-to-cell interactions and signaling pathways.
Key components of organoids
Organoids consist mostly of cell types, matrix and growth factors, and cytokines, which all work to build them up and function.
1. Cell source: For the organoid fabrication we need suitable cell sources such as pluripotent stem cells (embryonic stem cells and induced pluripotent stem cells) and organ-specific adult stem cells (intestinal stem cells). They can self-renew and differentiate into different cell types, and form the basis of organoids.
2. Basic medium: It is the heart of organoid culture and usually prepared using modified DMEM/F12 or another suitable medium. Different organoids might need different basal media. Retinal and gastric organoids might be cultured with a modified form of DMEM/F12; lung organoids might be cultured on RMPI-1640 medium.
3. Growth factors and cytokines: Growth factors and cytokines are what induces the growth and differentiation of organoids. There are epidermal growth factor (EGF), fibroblast growth factor (FGF), Wnt3A, R-Spondin-1, Noggin, etc. They proliferate, differentiate and remodel cells into tissues via signalling networks.
4. Mats and matrigel: Support organoids three-dimensionally. Other popular matrigels are Matrigel, which mimics the activity of mammalian cells' basement membrane and permits them to stick and differentiate.
6. Other Ingredients: Small molecules: like FGF4, MAPK inhibitors, etc. Such nanomoles can also control individual signalling pathways and drive the organoids' further growth and maturation.
The reasonable integration and tuning of these elements allows robust functions and complex organoids to be made – and will be valuable for disease studies, drug discovery and regenerative medicine.
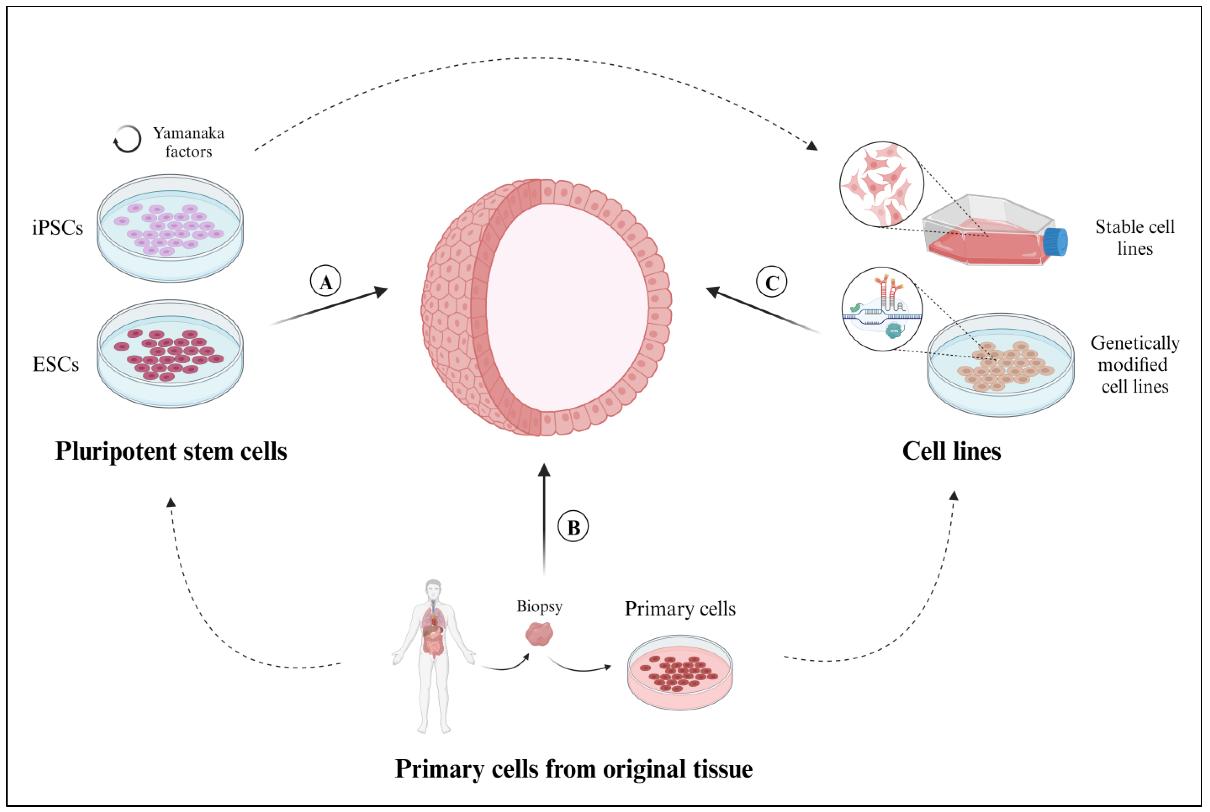 Figure 1 Sources of cells employed for the development of 3D organoid models. 1,4
Figure 1 Sources of cells employed for the development of 3D organoid models. 1,4
How to make organoids?
-
Cell sources selection: Organoids are generally made using donor stem cells or tissue samples from the human/animal body. They might be embryonic stem cells, pluripotent stem cells (PSC), or adult stem cells (like intestinal crypt cells). Culture of tissues – the brain, for example – is usually done with PSC when tissue isn't accessible.
-
Cell processing & culture medium preparation: The cells are washed, sheared and digested to remove impurities and release cells. Digestibility should be under control in 5-30 minutes, digestibility of hard tissue should not be over 1 hour. They float the cells in medium containing growth factors and small molecules. Those additives will be different for each organoid. This, for instance, is the hallmark of most tissue-based organoids.
-
3D culture: Cells are inserted into a support matrix like Matrigel to replicate the living environment. The three-dimensional scaffold of growth that matrigel offers cells lets them self-compose into organoids. While in the culture stage, growth factors (eg, EGF, FGF, etc.) need to be incorporated to induce the growth and differentiation of cells.
-
Culture conditions & environmental simulation: Generally, organoids are cultured under a special temperature and humidity in a cell incubator. In order to emulate the in vivo conditions, rotating bioreactors and gas-liquid interfaces (ALI) can be deployed. Also, organoids can be controlled in terms of growth and development via microporous arrays, microfluidic and other technologies.
-
Organoid maturation and identification: The maturation of organoids is a long process that may take weeks or even months. In the meantime, organoids become slowly distinct and develop organisational arrangements and activities like those of the body. We can detect adult organoids by immunofluorescence, H&E staining and more.
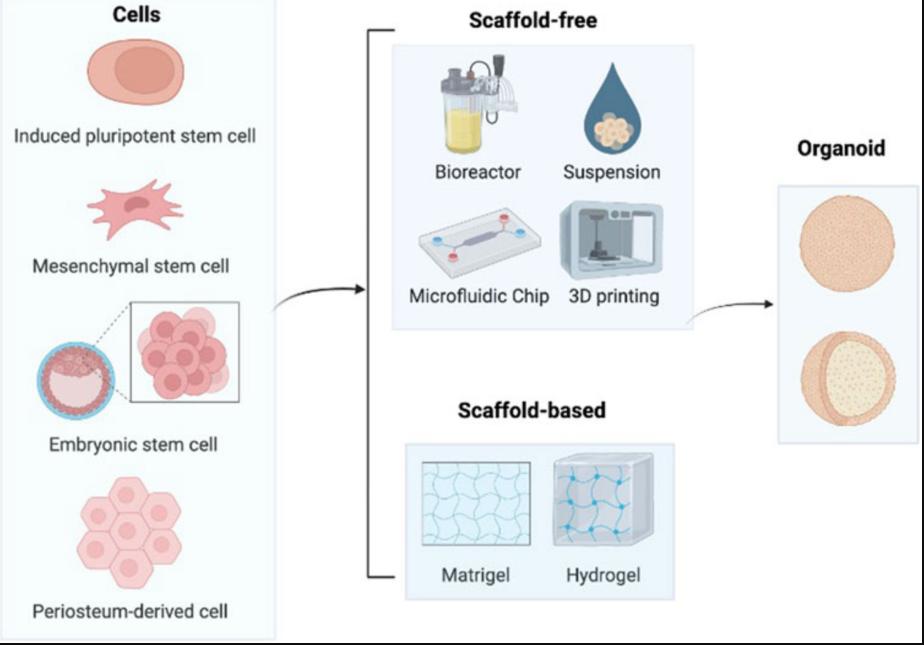 Figure 2. Construction process of bone organoids.2,4
Figure 2. Construction process of bone organoids.2,4
Making organoids is a complex and highly technical task that requires a combination of multiple technologies and methods to achieve. Successful organoid development requires not only suitable cell sources and culture media, but also precise environmental simulation and operation techniques.
Classification of Organoids
Organoids are categorized based on germ layer origin, cell source, developmental complexity, functional application, and culture methodology. Below is a detailed breakdown:
1. By Germ Layer Origin
Reflects embryonic lineage and tissue specificity.
|
Germ Layer
|
Organoid Types
|
Examples
|
Key Features
|
|
Endoderm
|
Gastrointestinal organoids, hepatic organoids
|
Intestinal organoids, Liver organoids, Pancreatic organoids
|
Epithelial polarization; crypt-villus structures (intestine)
|
|
Mesoderm
|
Renal organoids, cardiovascular organoids
|
Kidney organoids, Heart organoids, Blood Vessels organoids
|
Mesenchymal components (e.g., nephrons, cardiomyocytes)
|
|
Ectoderm
|
Neural organoids, epidermal organoids
|
Cerebral organoids, Retinal organoids, Skin organoids
|
Stratified layers (e.g., cortical neurons in brain organoids)
|
2. By Cell Source
Distinguishes between adult and pluripotent stem cell origins.
|
Cell Source
|
Organoid Types
|
Examples
|
Advantages/Limitations
|
|
Adult Stem Cells (ASCs)
|
Tissue-specific self-renewal organoids
|
Intestinal organoids, Liver organoids, Prostate organoids
|
Limited lineage potential; high genetic stability
|
|
Pluripotent Stem Cells (PSCs)
|
Directed differentiation models organoids
|
Cerebral organoids, Kidney organoids, Retinal organoids
|
Broad differentiation; models development/disease; ethical concerns (ESCs)
|
|
Directly Reprogrammed Cells
|
Induced lineage conversion organoids
|
Hepatocyte-like organoids, Neuron-like organoids
|
Bypasses pluripotency; faster generation; partial functionality
|
3. By Developmental Complexity
Stages reflect structural and functional maturation.
|
Stage
|
Characteristics
|
Examples
|
|
Pre-patterning
|
Initial cell aggregation; no tissue architecture
|
Early PSC-derived spheroids
|
|
Proto-organoids
|
Basic polarity; partial differentiation
|
Immature intestinal organoids
|
|
Mature Organoids
|
Functional units (e.g., crypts, nephrons)
|
Kidney with glomeruli-tubule systems
|
|
Vascularized
|
Endothelial integration; perfusion potential
|
Engineered liver/heart organoids
|
4. By Functional Application
Tailored for research, therapy, or industrial use.
5. By Culture Methodology
Techniques influencing physiological relevance and scalability.
Key Clarifications
-
Spheroids vs. Organoids:
-
Spheroids: Simple 3D aggregates (e.g., tumor spheroids) lacking self-organization.
-
Organoids: Self-organized, structurally/functionally complex (e.g., intestinal crypt-villus units).
-
Vascularization: Emerging techniques (e.g., co-culture with endothelial cells) enhance physiological relevance.
-
Gene Editing: CRISPR/Cas9-modified organoids enable precise disease modeling (e.g., CFTR mutations in cystic fibrosis).
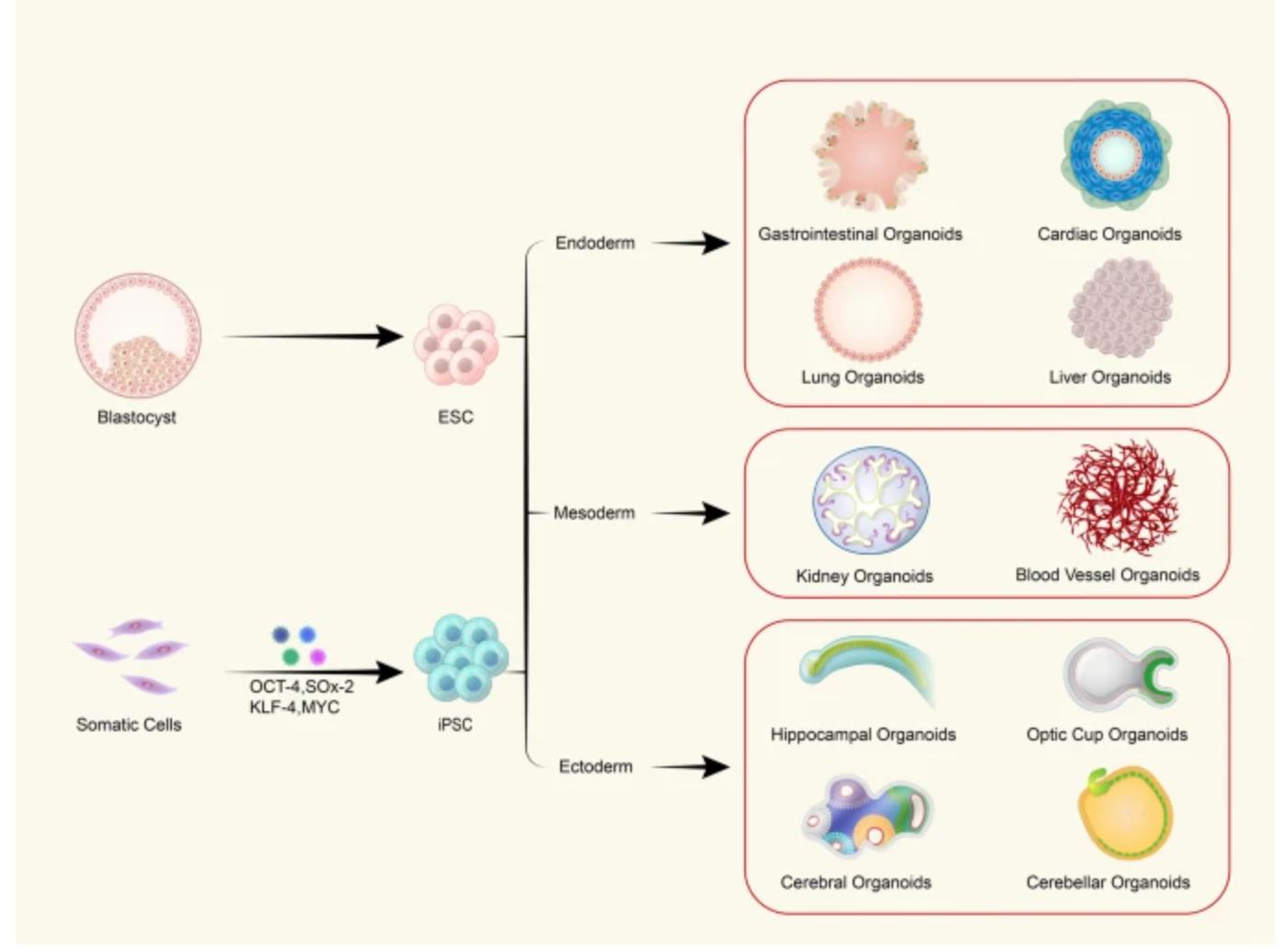 Figure 3. Schematic of the different organoids that can be derived from PSCs.3,4
Figure 3. Schematic of the different organoids that can be derived from PSCs.3,4
What are organoids used for?
Organoids are important biological models widely used in medical research and biotechnology
|
Application Area
|
Description
|
|
Disease Modeling
|
Simulate specific diseases (e.g., cancer, genetic disorders) to help understand their mechanisms.
|
|
Drug Development
|
Test the safety and efficacy of drugs, providing more accurate data on human responses.
|
|
Personalized Medicine
|
Develop personalized treatment plans based on organoids derived from patient cells.
|
|
Regenerative Medicine
|
Repair or replace damaged tissues or organs, promoting advancements in regenerative medicine.
|
|
Basic Research
|
Study cell interactions and responses to environmental changes, advancing fundamental biology.
|
|
Developmental Biology Research
|
Investigate key mechanisms in organ development and reveal causes of developmental abnormalities.
|
As an emerging in vitro model, organoids provide important tools for medical research and clinical applications. Their diverse applications make them valuable in the future of biomedical research.
What are the advantages of organoids?
There are several benefits of organoids that have caught more and more attention in biomedical research and clinical practice.
-
Highly realistic mimic the form and function of real organs.
Organoids can self-assemble in 3D environment, in vitro, to create tiny organ-like structures and even keep a few key functions of organs. 3D organoids can be used to better mimic cell differentiation, tissue architecture and organ function in live organisms enabling researchers to really gain a grip on the biology of organ development and function (especially in complex organs (brain, liver, kidney, etc.) ).
-
Overtaking of animal models and cell line models in nature.
Organoid technology overcomes the ethical and biological inconsistencies of standard animal experiments, compensating for the impossibility of 2-D cell culture. organoids are easier to work with, the modelling cycle is shorter, they are cheap, and can more precisely model human biological activity than animal models.
-
Personalized and precision medicine
The organoids could be made of patient cells that are also atypical, supporting the personalized medicine. In tumor research, for instance, patient derived tumor organoids (PDO) can be used to experiment with the effect of various drugs on tumour cells to decide which strategy will be best.
-
Decrease time for drug development and screening
Organoid can be used to make the process of drug discovery and screening much quicker, reveal how the drug acts in particular organs, and give insight into how to tailor a drug's performance and the way to treat it. Moreover, organoid models have high throughput and efficiency in drug screening.
-
Application of regenerative medicine
Organoid technology also has promise for regenerative medicine. It can be differentiated into specific cells that constitute target tissues through long-term culture and stability expansion for repair of damaged tissues or transplantation therapy. This approach is both safe and immune rejection-resistant.
-
Gene manipulation and research
Genes and disease pathways can be mapped onto organoid systems with today's genetic engineering techniques. By editing genes, for instance, scientists can check how organoids respond to particular gene mutations.
-
Ethical and cost advantages
Organoids are an in vitro culture technology without the risk of animal experiments, and organoids, as a non-invasive technique, are shorter in culture time and cheaper than animal and cell line models, as well as more economic benefit.
Creative Biolabs: Leading 3D Organoids Solutions for Cutting-Edge Research
Creative Biolabs offers a range of innovative 3d organoids related solution to enhance scientific research. Below is a concise list detailing some of our standout services and products:
3d organoids related services
3d organoids related Products
If you're interested in advancing your research with our state-of-the-art solutions, welcome to contact our support team.
References
-
Gómez-Álvarez, M.; Agustina-Hernández, M.; et al. Addressing Key Questions in Organoid Models: Who, Where, How, and Why? Int. J. Mol. Sci. 2023, 24, 16014. https://doi.org/10.3390/ijms242116014
-
Kong Y, Yang Y, Hou Y, Wang Y, et al. Advance in the application of organoids in bone diseases. Front. Cell Dev. Biol. 2024, 12:1459891. https://doi.org/10.3389/fcell.2024.1459891
-
Tang, XY., Wu, S., Wang, D. et al. Human organoids in basic research and clinical applications. Sig Transduct Target Ther 7, 168 (2022). https://doi.org/10.1038/s41392-022-01024-9
-
Distributed under Open Access license CC BY 4.0, without modification.
Research Model
Related Sections:

 Figure 1 Sources of cells employed for the development of 3D organoid models. 1,4
Figure 1 Sources of cells employed for the development of 3D organoid models. 1,4
 Figure 2. Construction process of bone organoids.2,4
Figure 2. Construction process of bone organoids.2,4
 Figure 3. Schematic of the different organoids that can be derived from PSCs.3,4
Figure 3. Schematic of the different organoids that can be derived from PSCs.3,4