Creating precise preclinical models is essential to enhance oncology drug development success because numerous promising drugs fail clinical trials due to traditional 2D cell cultures and in vivo animal models limitations. Tumor organoids represent a revolutionary advancement in 3D modeling which provides a more physiologically accurate system to study cancer and develop new therapies. Research interest in 3D tumor organoids has grown since 2009 because these models show potential to address the limitations of traditional models thereby transforming cancer research. Tumor organoids demonstrate widespread usefulness in cancer research and drug development which leads to the creation of more personalized and efficient treatment methods. Creative Biolabs summarizes tumor organoids knowledge to help researchers to accelerate their programs.
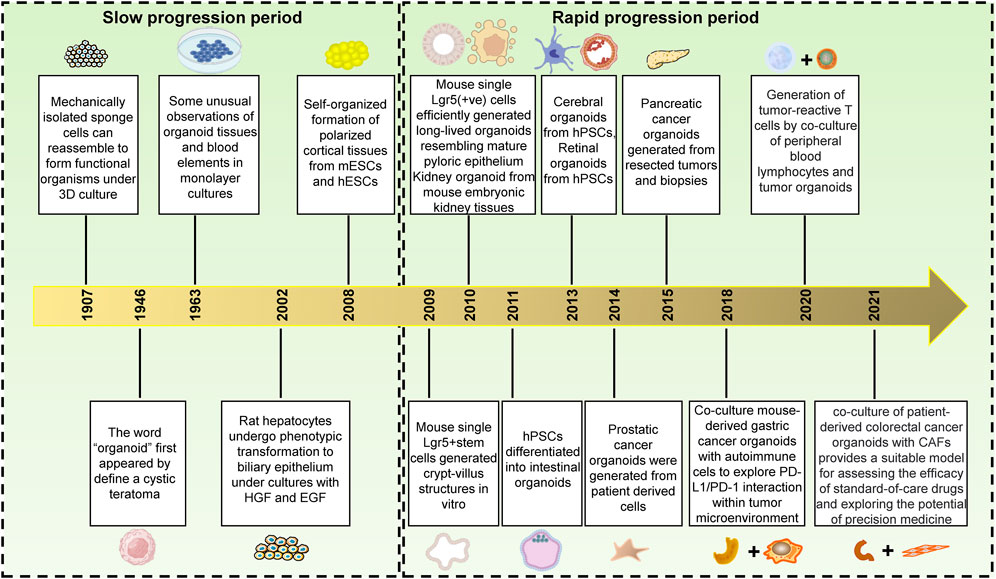 Figure 1. The history of organoids development.1,3
Figure 1. The history of organoids development.1,3
Tumor Organoids: Definition and Generation
The generation of tumor organoids from patient tumor samples or tumor stem cells results in three-dimensional cellular structures which self-organize and replicate the histopathological, genetic and phenotypic characteristics of the original tumor. During their generation process researchers use extracellular matrices like Matrigel to support tumor cell growth in a three-dimensional matrix in vitro which provides structural support and biochemical cues to facilitate cell organization. Culture media containing specific growth factors and nutrients supports cell survival alongside proliferation and differentiation which enables the simulation of in vivo conditions. Multiple sources including tissue biopsies and surgically excised tissues alongside circulating tumor cells known as liquid biopsies and tumor-derived stem cells provide origin points for tumor cells. Researchers can study metastatic disease progression and longitudinally assess treatment impacts by generating organoids from various cell sources including liquid biopsies.
Why Go 3D? Advantages and Limitations Compared to Traditional Models
Tumor organoids replicate the intricate three-dimensional structure of tumors more accurately than conventional 2D cell cultures which enables improved cellular communication and signaling fidelity. Tumor organoids preserve original tumor heterogeneity which proves vital for drug resistance studies while displaying enhanced clinical relevance and predictive power regarding drug response when compared to 2D cultures and animal models. Their scalability and manipulability are also beneficial for high-throughput drug screening and biomarker discovery. Compared to animal models, organoids offer reduced cost, shorter timelines, and fewer ethical concerns. They also exhibit genetic stability over extended culture periods.
However, basic organoid models may lack a fully developed vascular system, limiting nutrient and oxygen diffusion in larger structures. Vascularized organoids are being developed to address this. Some models may also lack a complete immune system and other components of the tumor microenvironment (TME) , but co-culture systems are emerging to better represent TME interactions. Challenges remain in standardizing and reproducing organoid culture protocols , and genetic/phenotypic drift over long culture periods requires monitoring.
Table 1: Comparison of Tumor Models
|
Model
|
Key Advantages
|
Key Limitations
|
|
2D Cell Culture
|
Simple and low-cost; Rapid growth; Amenable to high-throughput screening
|
Oversimplified model; Lack of 3D structure and heterogeneity; Limited clinical relevance; May induce genetic changes
|
|
Tumor Organoids
|
Recapitulates 3D structure and heterogeneity; Maintains cell function; Genetically stable; More clinically relevant than 2D; Suitable for high-throughput screening; Patient-derived potential; Reduced ethical concerns compared to animals
|
May lack full vascularization; Absence of complete immune system in basic models; Challenges in standardization and reproducibility; Potential for drift over long culture
|
|
Animal Models
|
Anatomical and physiological similarities to humans; Comprehensive systemic perspective; Versatile modeling through genetic manipulation
|
High cost and time; Ethical concerns; Often fail to accurately predict clinical efficacy; Species-specific differences
|
Unlocking Potential: Current Applications in Cancer Research and Drug Discovery
Tumor organoids are used extensively in disease modeling to study tumor initiation, progression, and metastasis. Organoids from precancerous lesions allow investigation of early events in malignancy. They are also powerful tools for preclinical drug screening to assess efficacy and toxicity, identify lead compounds, and evaluate drug combinations. Tumor organoid biobanks facilitate large-scale drug screens and the study of variable drug responses.
Patient-derived tumor organoids (PDTOs) are crucial for personalized medicine, enabling clinicians to potentially select the most effective treatment by testing drug responses on a patient's own tumor organoids. Integrating genomic and functional data from PDTOs helps identify therapeutic targets for precision medicine. Tumor organoids also aid in biomarker discovery for improved cancer management.
The Cutting Edge: Latest Advancements and Emerging Trends
The newest development in tumor organoid plaform focuses on combining co-culture systems to more accurately replicate the TME through the inclusion of immune cells along with fibroblasts and endothelial cells. Organ-on-a-chip microfluidic platforms create dynamic culture environments which allow for precise manipulation alongside real-time monitoring. Scientists are currently developing vascularized organoids that contain functional blood vessel networks. Researchers apply CRISPR-Cas9 gene editing technology to organoids for gene function analysis and targeted therapy development. Researchers use artificial intelligence (AI) to interpret the extensive datasets produced by organoid research.
Addressing the Inquiries: Common Questions and Concerns About Tumor Organoids
Scientists have succeeded in producing organoids from multiple types of carcinoma including those of colon, breast, lung, pancreas, ovary, head and neck, bladder, stomach, prostate, and liver. To establish organoids scientists first separate cells from tumor samples before growing them in an ECM-rich culture with designated growth factors. The successful establishment of organoids requires high-quality starting cells combined with proper ECM and culture medium alongside initial ROCK inhibitor application. Scientists successfully produced organoids from circulating tumor cells (CTCs) despite the difficulty to study metastasis. Scientists work to reduce clonal selection through the application of the same culture protocols for both normal and tumor tissues. The response of organoids to radiation and chemotherapy treatments allows scientists to study their effects. The research into creating stable co-culture systems including immune cells continues as an active field of investigation.
Key Applications of Tumor Organoids
Personalized Medicine
Tumor organoids derived from patient samples can be used to test individual responses to chemotherapy, targeted agents, and immunotherapies. This approach supports more tailored and effective treatment plans.
Drug Discovery and Development
By capturing tumor heterogeneity, organoids help identify novel drug targets, predict toxicity, and validate compound efficacy under physiologically relevant conditions.
Tumor Microenvironment Studies
Organoids can be co-cultured with immune cells, fibroblasts, or vasculature to study complex interactions within the tumor niche—essential for understanding immune evasion and resistance mechanisms.
Genetic and Functional Studies
CRISPR and other gene-editing tools can be applied to tumor organoids to explore the functional role of mutations, offering insights into oncogenic pathways and potential therapeutic targets.
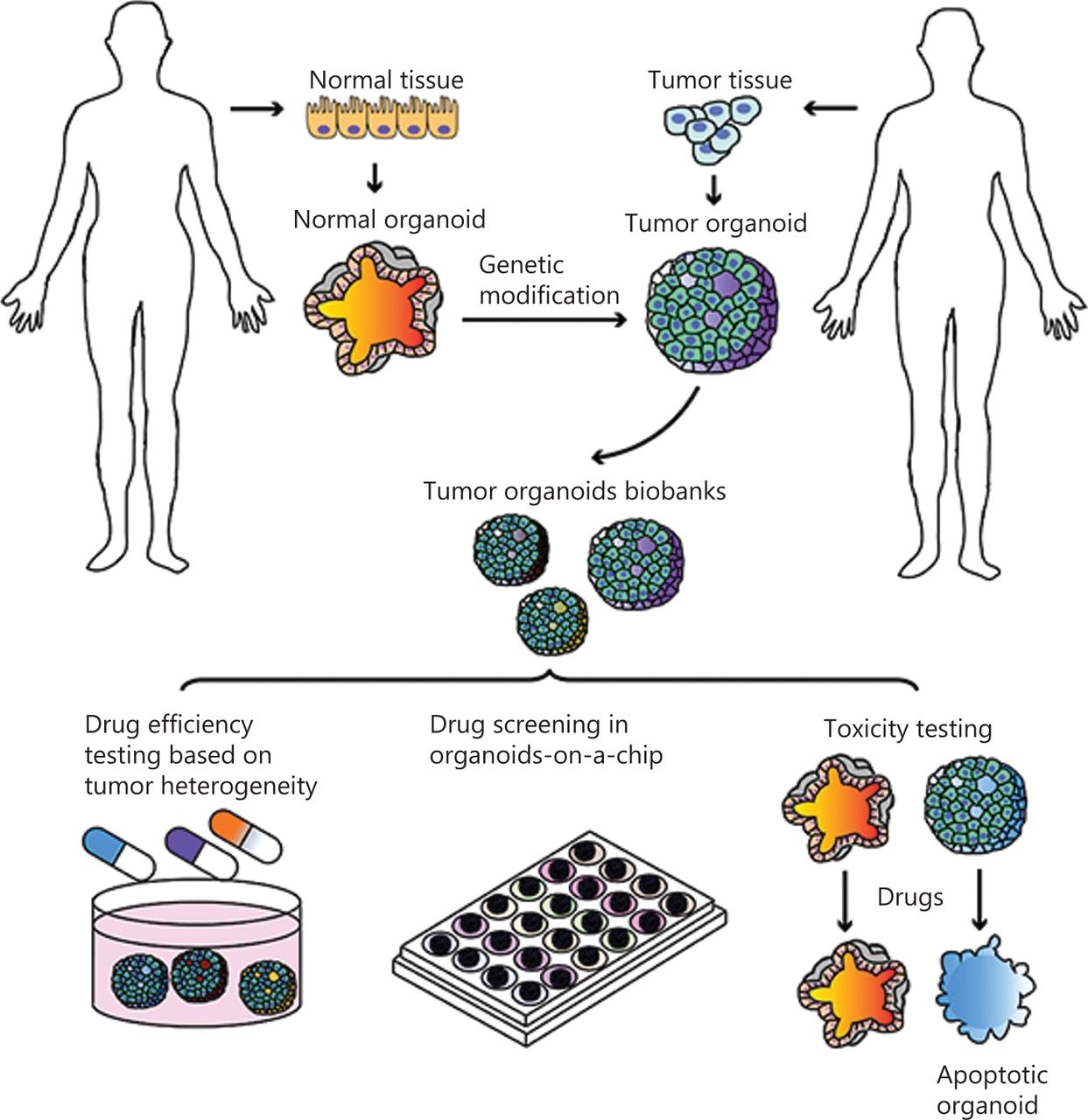 Figure 2. Organoids in cancer research and personalized medicine. 2,3
Figure 2. Organoids in cancer research and personalized medicine. 2,3
Future Perspectives and the Impact of Tumor Organoid Plaform
Tumor organoid plaform holds strong promise for personalized medicine by enabling the selection of drugs specific to patients and aiding the creation of targeted treatment options. Faster organoid culture methods, like microfluidic systems, are crucial for clinical translation. Research initiatives today work on creating sophisticated models that combine blood vessel networks with the immune system. Studies indicate that organoids offer a way to reduce reliance on animal testing. Applications of organoid plaform now expand to regenerative medicine and infectious disease research alongside its cancer research uses.
Tumor Organoids - A Promising Frontier in the Fight Against Cancer
Tumor organoids mark a new era in cancer research because they replicate human tumors with exceptional accuracy. Tumor organoids represent an evolving platform which improves cancer biology research and accelerates the creation of targeted medications with personalized treatment methods. The rapid advancements in tumor organoid capabilities establish them as a crucial resource for fighting cancer and developing personalized treatment options for patients.
FAQ
1: What types of cancers can be modeled using tumor organoids?
Tumor organoids have been successfully generated from a wide variety of carcinomas, including cancers of the colon, breast, lung, pancreas, ovary, head and neck, bladder, stomach, prostate, liver, and many others. Patient-derived tumor organoids can be established from most carcinomas.
2: How are tumor-derived organoids established, and what are the key considerations?
Tumor-derived organoids are typically established by isolating cells from tumor biopsies or resected tissue and resuspending them in an extracellular matrix (ECM) like Matrigel. These cells self-organize into 3D structures with medium changes every 2-3 days and passages every 7-10 days. Key considerations include using a ROCK inhibitor during the initial culture to prevent anoikis (a form of cell death), the quality and quantity of the starting tumor cells, and the appropriate choice of ECM and culture medium tailored to the specific cancer type.
3: How well do tumor organoids reflect the original tumor microenvironment?
Basic tumor organoid models do not possess a complete vascular system or complete immune system but maintain the original tumor's key histopathological, genetic, and phenotypic features. Scientists continue to develop sophisticated models by creating co-culture systems which combine immune cells with fibroblasts and endothelial cells to simulate the tumor microenvironment's intricate interactions. Scientists also create vascularized organoids which include functional networks of blood vessels.
Discover Creative Biolabs' Advanced Cancer Organoid Models
Enhance your oncology research with our state-of-the-art cancer organoid solutions tailored for precision medicine. Creative Biolabs offers a diverse portfolio, organized by model type, to help you accurately recapitulate tumor physiology and drive breakthrough discoveries.
Tumor Organoid Models
Patient-Derived Organoid Models
PDX-Derived Organoid Models
Elevate your cancer research with Creative Biolabs' innovative organoid platforms—contact us today to discover the ideal model for your study!
References
-
Jiang X, Oyang L, Peng Q, Liu Q, Xu X.; et al. Organoids: opportunities and challenges of cancer therapy. Front. Cell Dev. Biol. 2023, 11:1232528. doi: 10.3389/fcell.2023.1232528
-
Hui Yang, Yinuo Wang, Peng Wang.; et al. Tumor organoids for cancer research and personalized medicine. Cancer Biology & Medicine. 2022, 19 (3) 319-332; https://doi.org/10.20892/j.issn.2095-3941.2021.0335
-
Distributed under Open Access license CC BY 4.0, without modification.
Research Model
Related Sections:

 Figure 1. The history of organoids development.1,3
Figure 1. The history of organoids development.1,3
 Figure 2. Organoids in cancer research and personalized medicine. 2,3
Figure 2. Organoids in cancer research and personalized medicine. 2,3