Patient Derived Organoids Introduction
Patient Derived Organoids (PDOs) represent revolutionary three-dimensional (3D) models which have significant impact on biological research. These laboratory-created miniature tissue and organ models from patients faithfully reproduce both the cellular composition and structural intricacy observed in human bodies. PDOs maintain the unique genetic and molecular properties of their source individuals which allows them to serve as indispensable tools for the investigation of biological processes including development and disease. Researchers use Patient-Derived Tumor Organoids (PDTOs) as essential instruments to study tumor cell growth patterns along with differentiation processes and cellular interactions in cancer research. PDTOs create a microenvironment similar to that of a living organism which allows researchers to perform more precise drug testing and anticipate treatment responses in a patient's tumor. These models preserve the genetic makeup and physical characteristics of the original tumor tissue which helps researchers understand cancer progression and treatment reactions. PDTOs consist of small tumor models developed and maintained in laboratory conditions.
Tumor-Derived Organoids called tumouroids specifically originate from cancerous tissues although PDOs include multiple tissue types. These models are critical tools in examining tumor diversity which enables researchers to perform extensive tumor biology investigations and develop customized treatment plans. Over the last twenty years 3D cell culture techniques have developed from basic cell propagation methods into advanced models that maintain authentic physiological functions of cells. The shift towards 3D cell culture demonstrated that cells exhibit more genuine behavior and functionality when grown in spheroids or organoid structures. PDOs serve as a new research platform that connects classic in vitro experiments with in vivo animal research through their ability to model human organ structure and function on a small scale.
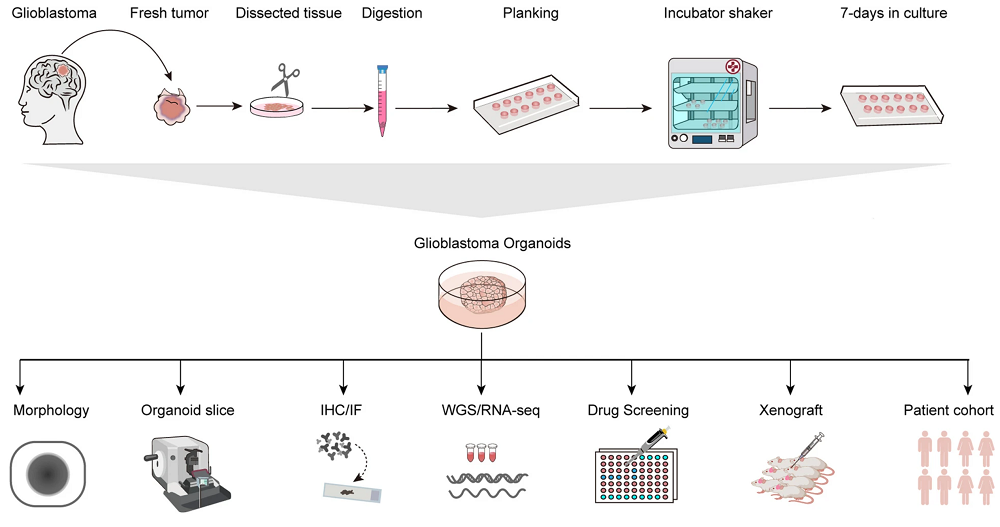 Figure 1. The GBM (Glioblastoma) organoids culture process and subsequent analysis are illustrated schematically.1,4
Figure 1. The GBM (Glioblastoma) organoids culture process and subsequent analysis are illustrated schematically.1,4
The Process of Generating Patient Derived Organoids
The detailed process of generating patient derived organoids begins by collecting tissue samples through biopsies or surgeries with informed consent. Research goals and tissue type differences dictate how subsequent steps in the process vary. First researchers commonly rinse tissue samples using nutrient-rich media before proceeding to mince them into tiny pieces. Scientists use enzymatic digestion to break down tissue further so they can isolate adult stem cells which form organoids. The vascular architecture remains better preserved when mechanical fragmentation techniques are applied to specific tissues such as glioblastoma. Researchers suspend isolated cells or tissue fragments in a specialized gelatinous protein mixture like Matrigel to provide structural support together with crucial growth factors for three-dimensional development. Small droplets of cell-Matrigel mixtures are created in petri dishes to enable solidification. Nutrients and growth factors are delivered through a customized liquid feed which supports the growth of developing organoids. The dishes undergo incubation in standard cell culture conditions which maintains a temperature of 37°C and 5% CO2. The organoids develop complex 3D structures during a period of about two weeks. The precise culture media composition together with specific niche factors determines the success of the process because they change based on the organoid type. The table below demonstrates that supplements required for PDTOs from various cancer types show significant variation.
|
Supplements
|
Breast cancer
|
Lung cancer
|
Liver cancer
|
Gall bladder adenoma/carcinoma
|
Head and neck cancer
|
Pancreatic cancer
|
Gastric cancers
|
Ovarian cancer
|
Cervical cancer
|
|
Base medium
|
DMEM
|
Advanced DMEM/F12
|
DMEM/F12
|
DMEM/F12
|
Advanced DMEM/F12
|
DMEM/F12
|
DMEM/F12
|
Advanced DMEM-F12
|
Advanced DMEM/F12
|
|
Antibiotic/Antimycotic
|
✓
|
✓
|
✓
|
✓
|
✓
|
✓
|
✓
|
✓
|
✓
|
|
L-Glutamine
|
✓
|
✓
|
✓
|
✓
|
✓
|
|
✓
|
✓
|
✓
|
|
HEPES
|
✓
|
✓
|
|
✓
|
✓
|
|
✓
|
✓
|
✓
|
|
Wnt-3a
|
✓
|
|
✓
|
✓/✗
|
|
✓
|
✓
|
|
|
|
R-Spondin
|
✓
|
✓
|
✓
|
✓/✗
|
✓
|
✓
|
✓
|
✓
|
✓
|
|
Noggin
|
✓
|
✓
|
✓
|
✓
|
✓
|
✓
|
✓
|
✓
|
✓
|
|
B27
|
✓
|
✓
|
✓
|
✓
|
✓
|
✓
|
✓
|
✓
|
✓
|
|
N2
|
|
|
✓
|
✓
|
|
|
|
|
|
|
Nicotinamide
|
|
✓
|
✓
|
✓
|
✓
|
|
✓
|
✓
|
✓
|
|
N-Acetyl-Cysteine
|
|
✓
|
✓
|
✓
|
✓
|
|
✓
|
✓
|
✓
|
|
Gastrin
|
|
|
✓
|
✓
|
|
|
✓
|
|
|
|
A83-01/ SB431542
|
✓
|
✓
|
✓
|
✓
|
✓
|
✓
|
✓
|
✓
|
✓
|
|
CHIR99021
|
|
|
|
|
✓
|
|
|
|
|
|
Y27632
|
✓
|
✓
|
✓
|
✓
|
|
✓
|
✓
|
✓
|
✓
|
|
EGF
|
✓
|
|
✓
|
✓
|
✓
|
✓
|
✓
|
✓
|
|
|
FGF2
|
|
|
|
|
✓
|
|
|
|
|
|
FGF7
|
|
✓
|
|
|
|
|
|
|
✓
|
|
FGF10
|
|
✓
|
✓
|
✓
|
✓
|
|
✓
|
✓
|
✓
|
|
HGF
|
|
|
✓
|
✓
|
|
|
|
|
|
|
IGF
|
|
|
|
✓
|
|
|
|
|
|
|
Hydrocortisone
|
✓
|
|
|
|
|
|
|
✓
|
|
|
Prostaglandin E2
|
|
|
|
✓/✗
|
✓
|
|
|
|
|
|
Forskolin
|
|
|
✓
|
✓
|
✓
|
|
|
✓
|
✓
|
|
FBS
|
|
|
✓
|
|
|
✓
|
|
|
|
Established organoids can be split and reseeded (sub-culturing or passaging) to create many identical copies. They can also be cryopreserved to form living biobanks, providing valuable resources for repeated studies without genetic compromise.
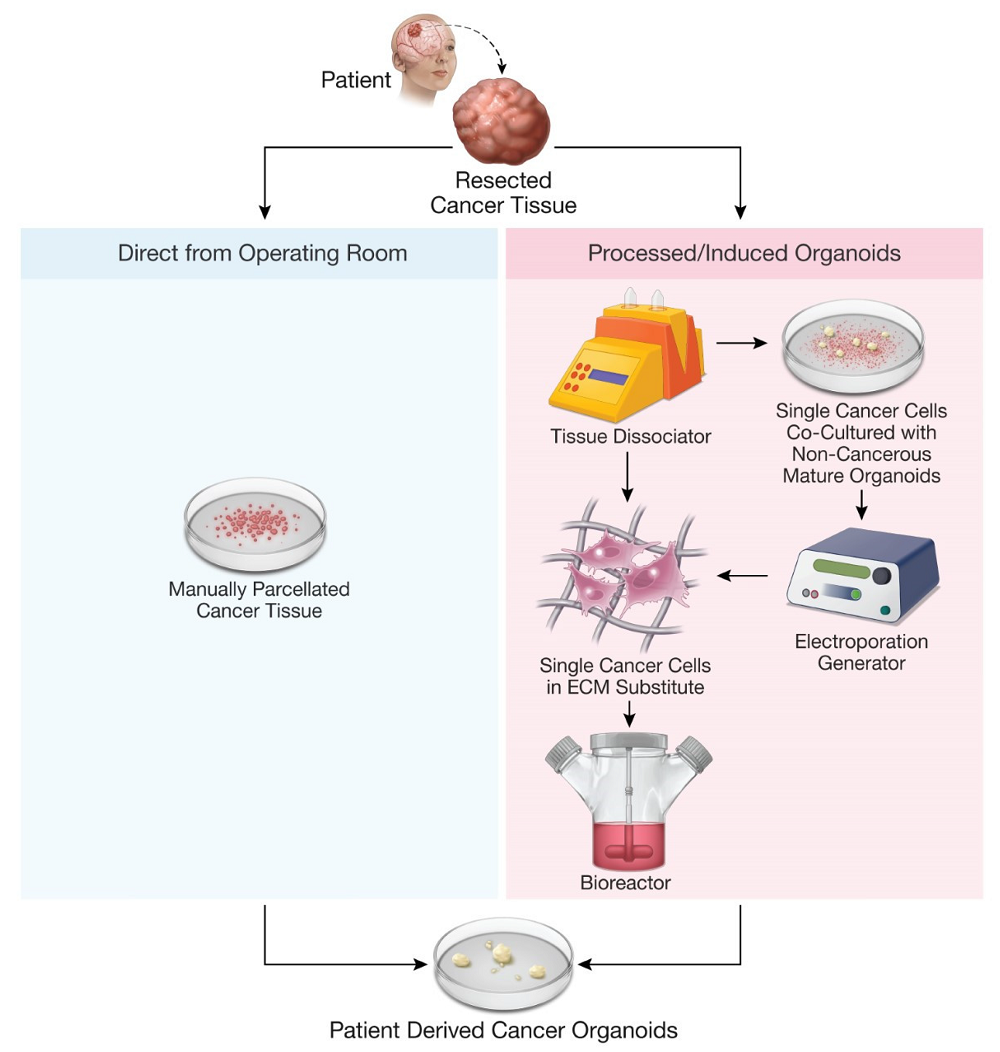 Figure 2. Methods for production of patient-derived organoids.2,4
Figure 2. Methods for production of patient-derived organoids.2,4
Diverse Applications of Patient Derived Organoids in Research
Cancer Research
In cancer research, PDTOs are exceptionally valuable for studying cancer biology due to their ability to replicate tumor structure and genetics. Researchers use them to investigate tumor growth, treatment responses, and to accelerate drug development.
-
PDTOs accurately predict patient tumor response to specific drugs, with studies showing high accuracy in various cancers.
-
These models enable the study of tumor heterogeneity, drug resistance mechanisms, and the identification of potential biomarkers.
-
Advancements allow the incorporation of immune system components into PDO cultures, facilitating the study of tumor-immune interactions for immunotherapy development.
Personalized Medicine
In personalized medicine, PDOs offer a unique advantage as they are derived from a patient's own tissue, ensuring genetic and molecular similarity. This allows for direct drug efficacy testing on the patient's organoids, streamlining the treatment selection process.
-
PDOs facilitate the identification of the most effective treatments for individual patients with conditions like cystic fibrosis and various cancers.
-
They maintain the genetic diversity found within tumors, enabling the development of highly personalized treatments targeting specific molecular vulnerabilities.
-
The use of "organoid scores" helps quantify drug responses, showing correlation with clinical outcomes and disease progression, thus guiding more informed treatment decisions.
Infectious Disease Modeling
PDOs are also proving invaluable in infectious disease modeling, offering a novel way to study human infections by replicating disease characteristics within the body. They allow researchers to investigate interactions between pathogens and human host tissues in various organs.
-
PDOs enable the study of how individual genetic variations influence responses to infections at a cellular level, paving the way for personalized prevention and treatment.
-
These models allow the investigation of pathogen interactions with a wide array of human tissues, overcoming limitations of animal models and traditional cell cultures.
-
Brain organoids, for example, have been successfully used to study infections like the Zika virus, demonstrating the utility of PDOs in understanding human-specific pathogen responses.
Regenerative Medicine
Beyond disease modeling, PDOs hold significant promise in regenerative medicine. By combining organoid systems with advanced biofabrication techniques, scientists are exploring the potential to replicate the complex functions of tissues and even entire organs.
-
Intestinal organoids are showing potential for therapeutic treatments for patients suffering from conditions like short bowel syndrome and human inflammatory bowel disease.
-
PDOs derived from a patient's own stem cells offer a unique platform for studying and potentially treating genetic disorders.
-
Bioengineering techniques allow precise control over the size, shape, and composition of organoids, contributing to the development of novel therapies for tissue repair and regeneration.
Advantages of Patient Derived Organoids Over Traditional Research Models
PDOs offer significant advantages compared to traditional research models.
Comparison to 2D Cell Cultures
-
More Physiologically Relevant: PDOs maintain key features of the original tissue, including structure and genetic heterogeneity, unlike 2D cultures that can lose these characteristics over time.
-
Improved Drug Response Prediction: PDOs offer better prediction of how drugs will affect a patient's condition compared to 2D cell lines.
-
Self-Organizing and Stable: PDOs spontaneously form complex 3D structures with multiple interacting cell types and exhibit genomic stability over long-term culture.
Comparison to Animal Models
-
Faster and More Cost-Effective: PDO models can be cultivated more quickly and are often less expensive than animal models like PDX.
-
Ethical Advantages and Human-Specific Studies: Using PDOs avoids ethical concerns of animal experimentation and allows for the study of human-specific biological processes.
-
Higher Throughput and Repeated Sampling: PDOs can be produced in larger quantities for efficient drug screening, and repeated samples can be obtained from the same patient over time.
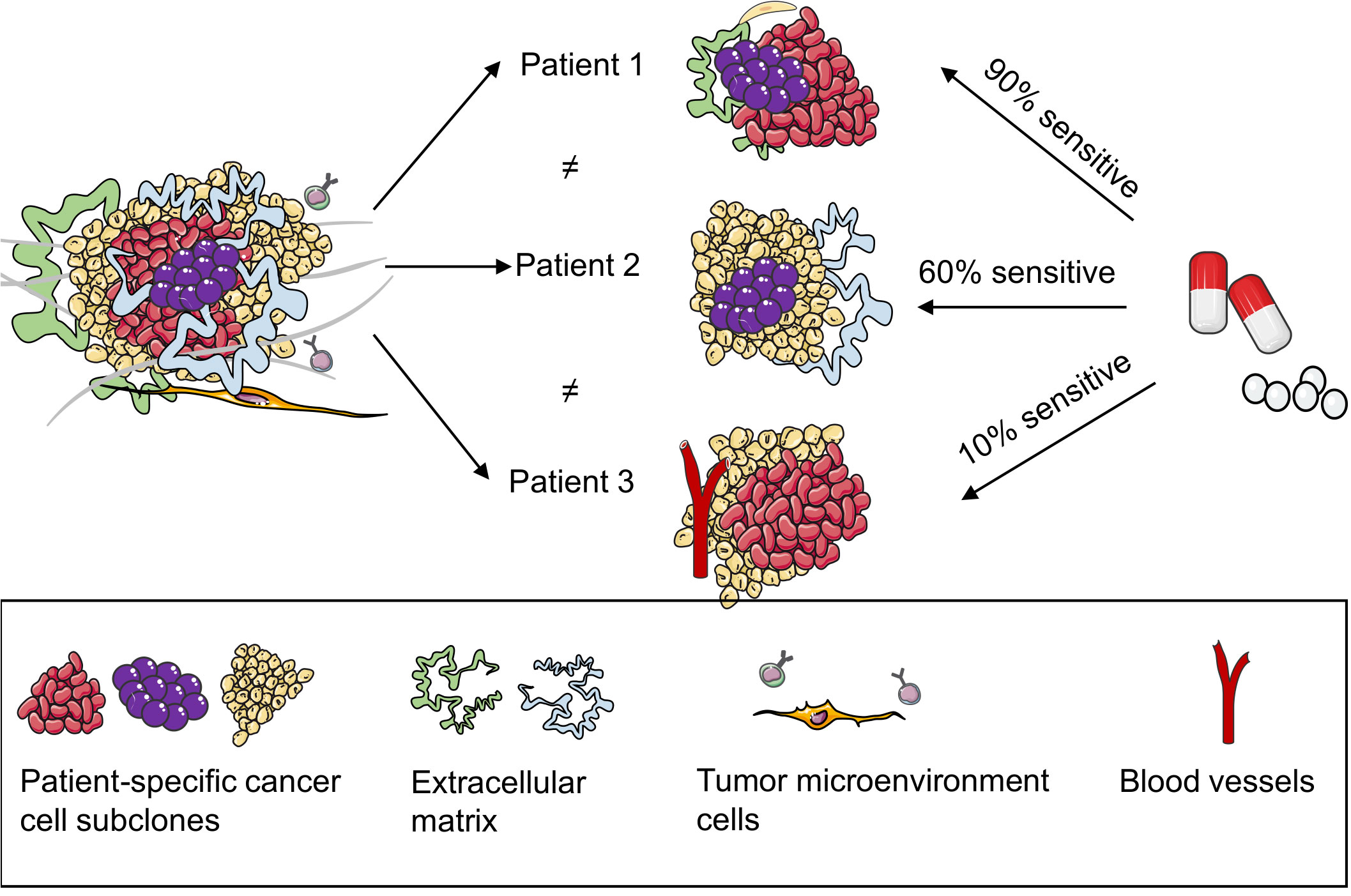 Figure 3. Patient-derived organoids (PDOs) can predict the responses of patients with breast cancer to drugs.3,4
Figure 3. Patient-derived organoids (PDOs) can predict the responses of patients with breast cancer to drugs.3,4
Limitations and Challenges of Patient Derived Organoids
Generating and maintaining PDOs can be complex and costly, with variable success rates depending on the tissue. They often lack the full complexity of the in vivo environment, missing crucial components like blood vessels and immune cells. Nutrient and oxygen diffusion can be limited in larger organoids, and standardization across different labs remains a challenge, impacting reproducibility.
Current Trends and Future Directions in Patient Derived Organoid Research
The field of PDO research progresses quickly as scientists work towards better culture systems alongside broadening clinical uses. Scientists are developing co-culture models incorporating immune cells along with stromal and endothelial cells to achieve more accurate in vivo condition simulations. Employing microfluidics and organ-on-a-chip platforms allows researchers to finely control microenvironments while 3D bioprinting enables large-scale production of uniform organoids. The development of standardized culture protocols alongside automated analysis tools will increase reproducibility and enhance drug response evaluation.
Advances in bioengineering and material science lead to better hydrogels and ECM-mimicking materials while commercial media becomes more consistent. Researchers are currently testing PDOs through clinical trials to guide cancer treatment while researchers investigate liquid biopsies as a less invasive tissue sourcing technique. Drug screening processes are speeding up through automation and advanced imaging technologies while regulatory agencies such as the FDA now acknowledge organoid-on-a-chip models more frequently in preclinical studies.
The latest research trends show single-cell analysis being used to investigate tumor heterogeneity while vascularized organoids advance drug delivery studies and CRISPR-Cas9 technology creates disease models and therapeutic targets. Research and clinical applications benefit from the creation of expansive PDO biobanks.
Conclusion: The Promising Future of Patient Derived Organoids
Patient Derived Organoids represent a major breakthrough in three-dimensional biological research and provide unique opportunities for personalized investigation into human health and disease. Patient Derived Organoids serve as critical tools in medical research and drug discovery because they replicate native tissue structure and function more effectively than traditional cell cultures and animal models. The development of advanced culturing techniques and bioengineering continues to overcome existing obstacles in cost efficiency and standardization while clinical translation expands their potential capabilities despite replication challenges of the complete in vivo environment. Patient Derived Organoids are expected to become essential tools for biomedical research and personalized medicine and drug discovery as research advances which will lead to better health outcomes for humans.
Explore Creative Biolabs' Comprehensive Cancer Organoid Portfolio
At Creative Biolabs, we understand that every research project is unique. Our range of cancer organoid models—developed from patient-derived samples and PDX sources—offers reliable and precise tools to mimic tumor microenvironments, helping you achieve breakthrough insights with ease. Choose from our carefully categorized offerings below:
Patient-Derived Organoid Models
PDX-Derived Organoid Models
Discover the model that perfectly matches your research requirements by reaching out to Creative Biolabs today!
References
-
Qu, S., Xu, R., Yi, G. et al. Patient-derived organoids in human cancer: a platform for fundamental research and precision medicine. Mol Biomed 5, 6 (2024). https://doi.org/10.1186/s43556-023-00165-9
-
Pernik, M.N.; Bird, C.E.; Traylor, J.I.; Shi, D.D.; Richardson, T.E.; McBrayer, S.K.; Abdullah, K.G. Patient-Derived Cancer Organoids for Precision Oncology Treatment. J. Pers. Med. 2021, 11, 423. https://doi.org/10.3390/jpm11050423
-
Shi Y, Guan Z, Cai G, Nie Y, Zhang C, Luo W and Liu J (2024) Patient-derived organoids: a promising tool for breast cancer research. Front. Oncol. 14:1350935. doi: 10.3389/fonc.2024.1350935
-
Distributed under Open Access license CC BY 4.0, without modification.
Research Model
Related Sections:

 Figure 1. The GBM (Glioblastoma) organoids culture process and subsequent analysis are illustrated schematically.1,4
Figure 1. The GBM (Glioblastoma) organoids culture process and subsequent analysis are illustrated schematically.1,4
 Figure 2. Methods for production of patient-derived organoids.2,4
Figure 2. Methods for production of patient-derived organoids.2,4
 Figure 3. Patient-derived organoids (PDOs) can predict the responses of patients with breast cancer to drugs.3,4
Figure 3. Patient-derived organoids (PDOs) can predict the responses of patients with breast cancer to drugs.3,4