What is Organ-on-a-Chip and 3D Models?
An Organ-on-a-Chip (OOC) is a multi-channel 3D microfluidic cell culture device that models the function, mechanics, and physiological response of an organ or an organ system. It consists of a microfluidic platform on which living human cells are integrated and the microenvironment is precisely controlled. Each OOC is constructed on a transparent polymer with hollow microfluidic channels that are lined with living human cells. The human cells are arranged to resemble the basic morphology of the target organ. The technology aims to create a more advanced in vitro substitute for complex tissues than cell culture, with the potential to replace animal models for drug development and toxin testing.
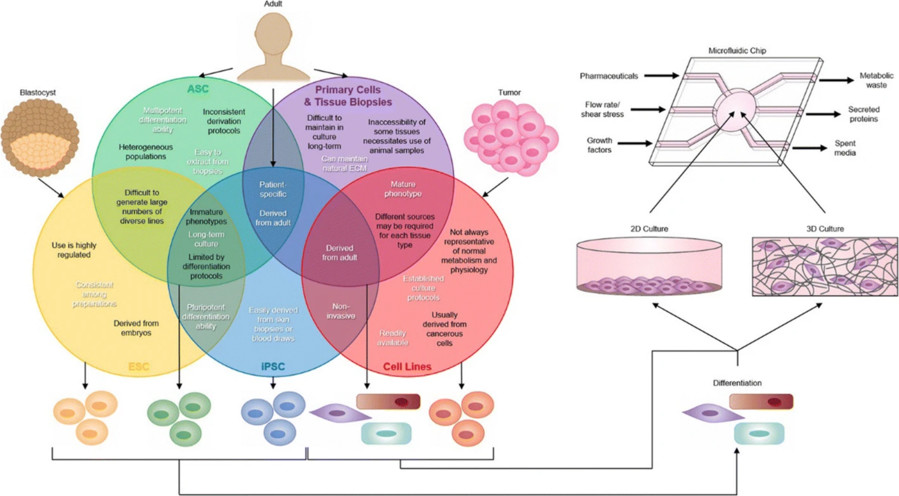 Figure 1 Tissue sources for the organ-on-a-chip (OOAC) devices.1,4
Figure 1 Tissue sources for the organ-on-a-chip (OOAC) devices.1,4
Historical Development of Organ-on-a-Chip
-
1980s - 1990s: Development of microfluidics began, primarily in the field of chemical analysis and diagnostics, which led to the birth of OOC concepts.
-
Early 2000s: First work demonstrating the ability to grow cells in microfluidic channels under flow conditions.
-
Late 2000s - Early 2010s: The true "Organ-on-a-Chip" concept, based on biomimicry of organ-level functions, took off.
-
2010 and beyond: This development sparked widespread interest and investment, and OOC became a fast-growing field of research and development. Numerous further efforts to build chips for liver, gut, kidney, heart, brain, and more.
-
Ongoing Vision: The long-term vision includes the ultimate goal of connected "human-on-a-chip" or "body-on-a-chip" systems, which will simulate systemic drug response.
3D models of cells can be achieved through three-dimensional cell culture. 3D cell culture models, sometimes called 3D cell models, are three-dimensional cell culture systems that extend the two-dimensional (2D) cell culture systems. 3D cell models can be constructed in different ways. One method is to create a 3D cell culture system, another is to create organoids, and another is 3D bioprinting. The architecture of 3D cell culture system is more complicated than 2D cell culture system. The 3D cell culture system has a flexible ECM gel which can support cell-shape changes and cell-cell connections, which was not allowed in 2D culture substrates. Even if 3D cell culture system is perfect, it cannot exactly mimic the cellular properties of an organ, for example, tissue-to-tissue interactions, spatial-temporal chemical gradients, and mechanically active microenvironments.
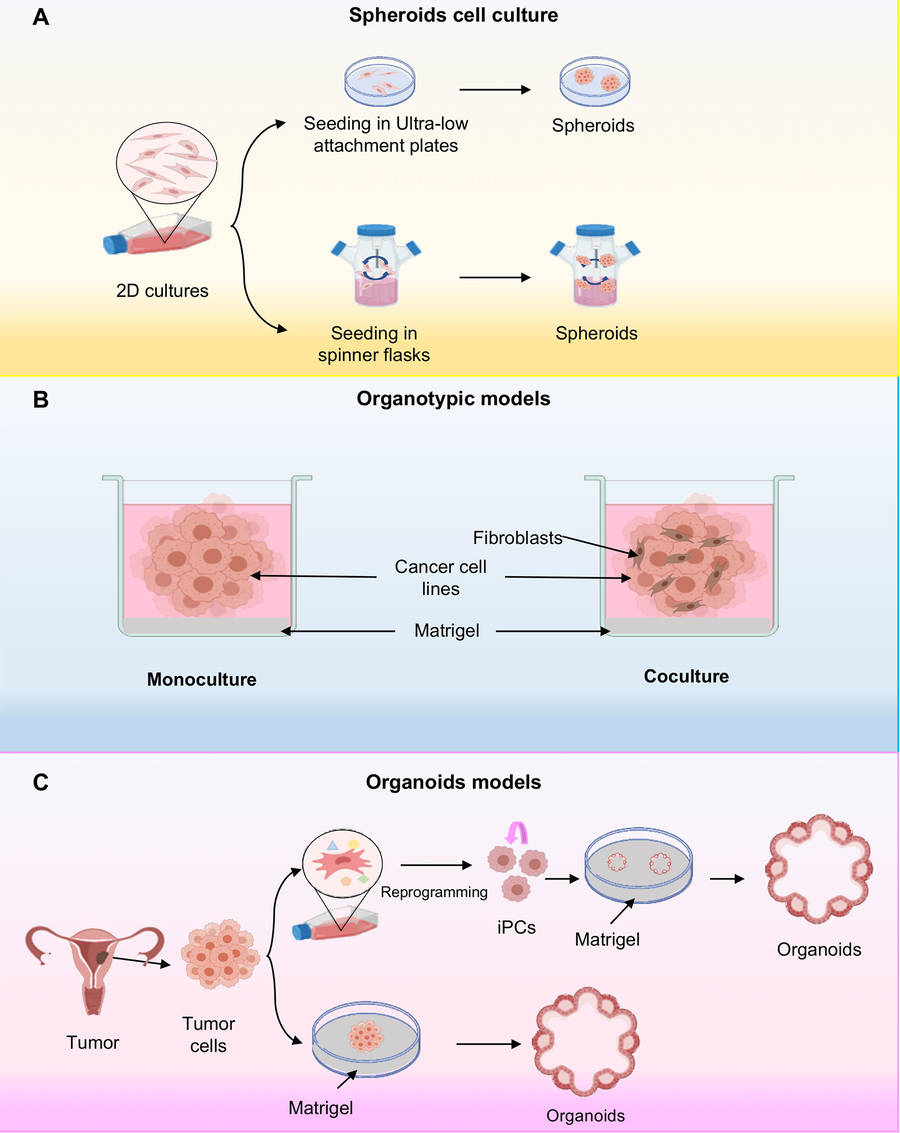 Figure 2 Three-dimensional cell cultures.2,4
Figure 2 Three-dimensional cell cultures.2,4
|
Model Type
|
Description & Key Characteristics
|
Advantages
|
|
Spheroids
|
Spherical cell aggregates formed through self-assembly, mimicking basic tissue features like gradients. They can be single or multicellular, often used to model tumors.
|
Cancer research (drug screening, understanding resistance), drug discovery, and tissue development studies.
|
|
Organotypic Models
|
3D organ-like structures that promote differentiation and resemble native tissue structure and function. They often involve co-cultures and specific matrices or interfaces.
|
Modeling specific tissues (skin, lung, gut), studying disease mechanisms (e.g., cancer microenvironment), toxicity testing, and cell-cell communication.
|
|
Organoids
|
Self-organizing 3D tissues derived from stem cells (adult or induced pluripotent), replicating the functional features and spatial organization of real organs. They grow in a supportive matrix and maintain long-term culture.
|
Disease modeling (cancer, neurological, developmental), drug discovery and personalized medicine (patient-derived organoids), regenerative medicine, organ development studies, and virology.
|
|
Other Emerging 3D Models
|
This category includes advanced models like Organs-on-a-chip (microfluidic systems with controlled environments), Bioprinted models (customized tissue structures), and Decellularized tissues (natural scaffolds). They offer more precise control and complexity.
|
Advanced drug screening and ADME studies, studying organ-organ crosstalk, tissue engineering, and personalized medicine (e.g., "tumor-on-a-chip").
|
Microfluidic Platforms for Organs-on-Chip
The fundamental basis of Organ-on-a-Chip technology is the microfluidic platform. A network of microchannels (typically made from PDMS or other biocompatible polymers) in a microfluidic platform can control the delivery of nutrients, oxygen, and biochemical signals to cells, and establish well-defined concentration gradients in the microfluidic channels that better mimic the in vivo microenvironment. Sensors and actuators can be integrated into the microfluidic platforms to monitor and control cell responses in real-time. Some of the Organ-on-a-Chip devices include electrodes for measuring electrical activity of cardiac cells or mechanical actuators to apply cyclic strain to cells to mimic the mechanical forces cells experience in the body (e.g. lung cells being stretched during breathing).
3D Cell Culture Techniques
In contrast to the two-dimensional (2D) monolayer culture, 3D cell culture systems attempt to overcome these limitations by allowing cells to grow in a scaffold-free or scaffold-based 3D environment. This approach results in structures with a more physiologically relevant cell-cell and cell-ECM interactions.
|
Technology Type
|
Representative Method
|
Application
|
|
Scaffold-Free
|
Magnetic levitation culture, Rotary bioreactors
|
High-throughput tumor spheroid generation
|
|
Scaffold-Based
|
Hydrogels (e.g., Matrigel), Synthetic polymers
|
Refined organoid structures
|
|
Bioprinting
|
4D thermo-responsive hydrogel printing
|
Cardiac organoids
|
Advantages and Disadvantages of Organs-on-Chip and 3D Models
Both OOC and 3D models represent significant advancements over 2D cultures, but they each possess distinct advantages and disadvantages that dictate their suitability for various research and commercial applications.
|
Feature
|
Organ-on-a-Chip (OOC) Models
|
3D Cell Culture Models (Spheroids, Organoids, Scaffold-based)
|
|
Advantages
|
- Physiological Environment: Physiological flow, shear stress, cyclic strain, chemical gradients
- Physiological Tissue-Tissue Interfaces: Highly defined interfaces (e.g. epithelial-endothelial barriers)
- Precise Control & Monitoring: Control delivery of stimuli, real-time non-invasive monitoring
- Reduced Animal Testing: Generate more human-relevant data, less animal use
|
- Additional Physiologically Relevant Structures: Mimics in vivo structures, cells/ECM interactions, cell morphology.
- Nutrient/Oxygen Gradients: Mimics in vivo, generate heterogeneous cell populations (e.g. tumor models).
- Organoids for Developmental & Disease Modeling: Powerful for studying human development, genetic disorders, patient-specific responses.
- ECM Mimicry: Scaffold-based cultures can control the ECM properties.
|
|
Disadvantages
|
- High Complexity & Fabrication: Typically, hard to design, fabricate, and operate. Requires special facilities.
- Standardization Challenges: Difficult to standardize because of different designs and protocols.
- Scalability for High-Throughput: Difficult to achieve true HTS for complex OOC systems.
- Replication of Full Organ Complexity: Simplified representations. Don't attempt to replicate the full complexity of an entire organ.
|
- Less Dynamic Flow/Mechanical Stimuli: Static 3D cultures lack physiological flow, shear stress, or cyclic strain.
- Less Inter-Organ Communication: Typically consist of single organs and cannot easily model systemic interactions.
- Less Variable Organoid Culture: More variable from batch-to-batch.
- Less Precise Environmental Control: More difficult to maintain highly specific and stable chemical gradients or precise mechanical stimuli as in OOCs.
|
Key Applications of Organs-on-Chip and 3D Models
Both Organ-on-a-Chip (OOC) and 3D cell culture models are rapidly transforming several critical areas of biomedical research and have substantial implications for pharmaceutical and biotechnology industries.
-
Patient-specific Drug Screening
Patient-derived tumor organoids from biopsies or iPSCs can be grown and tested for various treatments. This is known as "patient-in-a-dish". "Patient-specific" drug screening allows the researchers to customize treatment for each patient rather than treat everyone the same. For example, colon cancer organoids have a high concordance with chemotherapy clinical response.
-
Cell-cell and Cell-ECM interactions
3D cultures help to examine cell-cell and cell-ECM interactions. These interactions control tissue organization, differentiation and function. 3D cultures help to explore the role of cell-cell and cell-ECM interactions in health and disease.
-
Disease Modeling
Patient derived OOCs and organoids can be used to simulate genetic diseases with patient specific gene mutations and cellular microenvironments (neurological diseases such as cystic fibrosis, Alzheimer's or Parkinson's), in order to better understand disease mechanisms and generate new therapies.
-
Organ Physiology
OOC with a precisely defined microenvironment can summarize the complex organ physiology in the body, including barrier function, immune cell transport, and intercellular communication. Researchers can replicate physical and chemical clues to define their role in maintaining homeostasis in the body.
Conclusion
On chip organization (OOC) and 3D cell culture models may completely change the existing 2D cell culture model space, surpassing its limitations. OOC devices can replicate organ level physiology in microfluidic devices, such as flow, mechanical forces, and tissue interfaces, providing a well-controlled environment for drug ADME, toxicity, and disease modeling (such as chip lungs for ARDS). 3D cell cultures (spheres, organoids) can replicate tissue structures and cell-cell interactions in vivo for better physiological relevance and drug screening scale (such as tumor organoids for personalized oncology). OOC provides better dynamic control and interfaces with other organs, while 3D models are easier to develop for high-throughput applications. Both can reduce animal use, accelerate drug discovery, and provide personalized medicine with more health and disease prediction models related to humans.
Overview of What Creative Biolabs Can Provide
Creative Biolabs is one of the first biotechnology companies to implement the Organ-on-a-Chip and 3D Cell Culture technologies for the support of drug discovery and preclinical research within pharmaceutical, biotechnology and academic sectors.
We are dedicated to improving our state-of-the-art scientific research and providing cutting-edge in vitro solutions for our clients. Let us help you shorten your drug discovery timeline and expand your understanding of human biology and disease.
Frequently Asked Questions of Organ-on-a-Chip
Q: How do Organ-on-a-Chip models improve drug toxicity testing?
A: The OOC model improves toxicity testing as it provides more accurate predictions of drug-induced organ damage in the context of human related models. For example, Liver-on-a-chip can evaluate liver toxicity by simulating metabolic activity and bile secretion, providing more translatability than animal models or static 2D cultures. In addition, strict control over drug concentration and exposure time allows for dose-response studies.
Q: How do Organ-on-a-Chip models differ from 3D cell cultures like organoids?
A: Although both have improved 2D cultivation, the OOC model has the additional ability to control microfluidic flow and mechanical forces, which enables the model to simulate dynamic microenvironments and tissue tissue interfaces, and strictly control flow velocity, shear stress, and chemical gradients. 3D cell cultures, such as spheres and organoids, often focus more on reconstructing tissue structures and intercellular interactions in static or semi static environments, with little continuous flow or active mechanical stimulation. However, hybrid power systems that combine the characteristics of both are emerging.
Q: What are the future development directions for Organ-on-a-Chip?
A: In the future, it is likely to move towards higher throughput and multi-organ integrated modeling to better simulate the complex physiological processes of the human body. Technological improvements may also enable it to play a greater role in personalized medicine.
References
-
Wu Q, Liu J, Wang X, et al. Organ-on-a-chip: recent breakthroughs and future prospects. Biomedical engineering online, 2020, 19: 1-19. https://doi.org/10.1186/s12938-020-0752-0
-
Salinas-Vera Y M, Valdes J, Perez-Navarro Y, et al. Three-dimensional 3D culture models in gynecological and breast cancer research. Frontiers in Oncology, 2022, 12: 826113. https://doi.org/10.3389/fonc.2022.826113
-
Distributed under Open Access license CC BY 4.0, without modification.
Research Model
Related Sections:

 Figure 1 Tissue sources for the organ-on-a-chip (OOAC) devices.1,4
Figure 1 Tissue sources for the organ-on-a-chip (OOAC) devices.1,4
 Figure 2 Three-dimensional cell cultures.2,4
Figure 2 Three-dimensional cell cultures.2,4