Organ-on-a-Chip Definition
Organ-on-Chip (OOC) is a multi-channel 3D microfluidic cell culture integrated circuit (chip) that simulates the activity, mechanics, and physiological responses of the entire organ or organ system. It constitutes an important topic of biomedical engineering research, more precisely in the field of biological MEMS. The integration of lab-on-a-chip (LOC) and cell biology makes it possible to study human physiology in the context of specific organs. By serving as more complex in vitro approximations of complex tissues than standard cell cultures, they offer the potential as alternatives to animal models for drug development and toxin testing. Although many publications claim to have translated organ functions into this interface, the development of these microfluidic applications is still in its infancy. The design and methods of organs on chips vary among researchers. The organs simulated by microfluidic devices include the brain, lungs, heart, kidneys, liver, prostate, blood vessels (arteries), skin, bones, cartilage, etc.
What are Traditional Models?
Traditional models in biomedical research primarily encompass two broad categories: two-dimensional (2D) cell cultures (in vitro) and animal models (in vivo).
Two-Dimensional (2D) Cell Cultures: Cell culture or tissue culture is the process of cell growth under controlled conditions, typically outside of natural environments. After isolating the cells of interest from living tissue, they can subsequently be maintained under strictly controlled conditions. They require an environment to maintain body temperature (37 ° C) within the incubator. Conditions are different for different cell types, but are usually a suitable container for substrates or nutrient rich culture media (amino acids, carbohydrates, vitamins, minerals), growth factors, hormones and gases (CO2, O2), and a jointed environment (pH buffer, osmotic pressure, temperature). Cells in culture usually need a surface or artificial matrix on which to form a monolayer (one cell thick) adherent culture, but some cells can grow while freely floating in suspension medium.
 Figure 1 Two-dimensional models to study ICD.1,3
Figure 1 Two-dimensional models to study ICD.1,3
Animal Models (in vivo): Animal models (short for animal disease models) are living, non-human, typically genetically engineered animals used to study and investigate human diseases with the aim of better understanding disease processes without harming humans. Although the biological activity in animal models cannot guarantee its impact on humans, many drugs, treatments, and cure methods for human diseases have been developed under the guidance of animal models. In the study of developmental processes, animal models representing specific taxonomic groups are also referred to as model organisms. There are three main types of animal models: homologous, isomorphic, and predictive. Homologous animals have the same etiology, symptoms, and treatment options as humans with the same disease. Isomorphic animals only have the same symptoms and treatment methods. The predictive model is only similar to specific human diseases in a few aspects. However, these mechanisms are useful in separating and predicting a set of disease characteristics.
Traditional Models vs. Organ-on-a-Chip
The contrast between traditional models and OOC technology illuminates the transformative potential of the latter in addressing long-standing challenges in biomedical research.
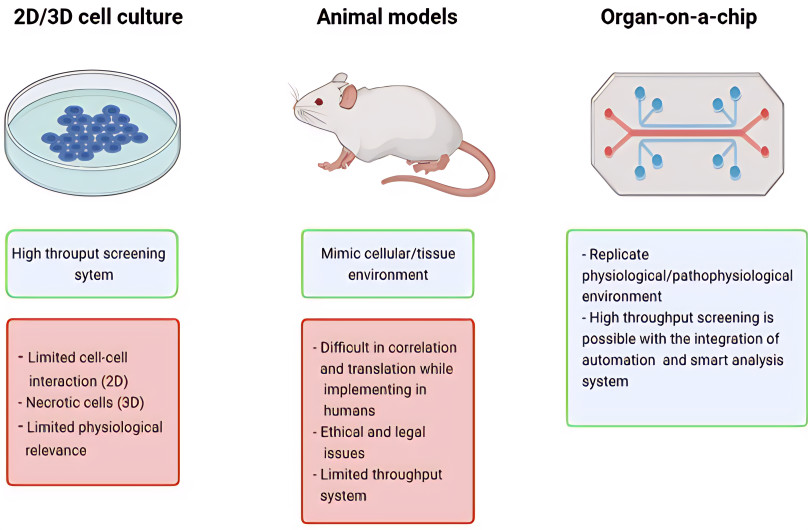 Figure 2 Some of the advantages of OOAC technology over cell cultures and animal models.2,3
Figure 2 Some of the advantages of OOAC technology over cell cultures and animal models.2,3
-
2D Cell Culture vs. Organ-on-a-Chip
It is now widely believed that 3D cultures are more representative of the in vivo microenvironment than 2D models. The heterogeneity characteristics of TME in vivo were clearly reflected through the use of heterocellular 3D culture. Reconstructing intercellular contact, achieving true expression of molecules (such as adhesion molecules, cytokines, growth factors, etc.), forming hypoxic cores and diffusion gradients, all of which have a significant impact on tumor behavior and treatment response. For example, the presence of intercellular contact strongly affects the response of cancer cells to cell death stimuli; If cells are seeded in a 3D model, the response rate is much lower.
-
Animal Testing vs. Organ-on-a-Chip
Animal models provide a systemic context but face translational challenges due to species differences. Many drugs succeed in animal studies yet fail in human trials, with failure rates sometimes surpassing 90% in certain therapeutic areas. This attrition is costly, time-consuming, and ethically concerning. A notable example is thalidomide, which caused birth defects in humans but passed safety tests in several animal models, underscoring critical species variability in drug response.
|
Feature
|
Animal Testing
|
Organs-on-Chips
|
|
Ethical Concerns
|
High
|
Lower
|
|
Cost
|
High
|
Lower
|
|
Relevance to Humans
|
Limited
|
Higher
|
|
Complexity
|
High
|
Can replicate some aspects, but still developing to fully represent full organ/body complexity
|
|
Speed
|
Slower
|
Faster
|
|
Regulatory Acceptance
|
Established
|
Growing, but still developing
|
Organ-on-a-Chip Market Analysis and Forecasts
Chip organ technology is under development as a potential alternative to animal drug testing. Due to differences in physiology and drug metabolism between animals and humans, drugs in the final stage of preclinical trials often fail. The high consumption of potential candidate drugs and ethical concerns regarding drug testing on animals have led to the development of organ chip technology to reduce dependence on animals and improve the efficacy of drug toxicity testing. Animals have been prohibited for cosmetic testing in Europe since 2013. The US Food and Drug Administration passed the Modernization Act 2.0 in 2023 to lessen the dependence on animals for drug testing and further focus on other types of drug testing such as organ chips.
The challenges that could obstruct OOC technology advancement into the clinical drug development stage include the limited renewable source of cells which could compromise the reliability of models, ethical issues for certain types of cells such as induced pluripotent stem cells (iPSCs) and difficulties in biological scaling to mimic human organs' complexity. The low throughput of current OOC technology affects the scalability and efficiency of the model, hindering its integration into clinical research. To address these limitations, implementing parallelization and automation in OOC systems, namely high-throughput OOC (HT-OOC) technology, provides a promising solution for advancing drug development beyond the preclinical stage, facilitating comprehensive testing of large compound libraries to determine relevant drugs for disease pathogenesis.
The future of the OOC market appears very promising, driven by its potential to overcome limitations of traditional models, meet ethical demands, and ultimately accelerate the development of safer and more effective therapies.
Organ-on-a-Chip Commercialization
From the process of transformation and commercialization of organ chips, the industrial point of view is taken into account first, such as the technical standardization and reliability, operability and convenience, price, and legality. Therefore, for the transformation and commercialization of these chips, further analysis and verification are required to confirm their diagnostic and therapeutic efficacy and reproducibility and to meet the practical needs of pharmacology and medical application as well as the requirements of FDA regulations. No matter at which stage of the organ chips, close communication and coordination between academic institutions, industrial R&D departments, and medical health organizations in the early stages can establish a positive feedback loop. It is to confirm the effectiveness of this platform and obtain its maximum value in the healthcare industry. Despite the enormous potential of organ chip technology, a major challenge for its commercialization is insufficient venture capital funding. According to public data such as Crunchbase, only a few organ chip startups have received significant investment. To overcome this challenge, the national healthcare and research system can play an important role, such as promoting cooperation with academic institutions and businesses, providing intellectual property protection support, and offering funding channels.
Overview of What Creative Biolabs Can Provide
Creative Biolabs is a pioneering force in the Organ-on-a-Chip landscape, offering a comprehensive suite of services and products designed to accelerate biomedical research and drug development. Our expertise spans the entire OOC workflow, from custom chip design and fabrication to advanced cell culture and comprehensive analytical services.
Organ-on-a-Chip models, including but not limited to:
-
Lung-on-a-Chip: For studying respiratory diseases, drug inhalation toxicology, and viral infections.
-
Liver-on-a-Chip: Ideal for drug metabolism studies, hepatotoxicity assessment, and modeling liver diseases like NAFLD/NASH.
-
Kidney-on-a-Chip: Essential for nephrotoxicity screening and modeling renal diseases.
-
Intestine-on-a-Chip: For absorption studies, gut microbiome interactions, and inflammatory bowel disease research.
-
Brain-on-a-Chip: For neurotoxicity, neurodegenerative disease modeling, and blood-brain barrier studies.
-
Heart-on-a-Chip: For cardiotoxicity screening, cardiac disease modeling, and regenerative medicine applications.
At Creative Biolabs, our commitment to cutting-edge research ensures that our OOC platforms are robust, reproducible, and highly translational, empowering our partners to make more informed decisions in their preclinical pipelines and ultimately bring safer and more effective therapies to patients faster.
Frequently Asked Questions about Organ-on-a-Chip
Q: How do Organ-on-a-Chip models compare to 3D spheroids or organoids?
A: While spheroids and organoids are valuable 3D cell culture models, OOCs offer superior control over the microenvironment. OOCs uniquely incorporate dynamic fluid flow, mechanical stimuli, and the ability to precisely define tissue-tissue interfaces, which are often lacking in static spheroids/organoids, thus providing a more faithful recreation of in vivo physiology.
Q: Are Organ-on-a-Chip models fully replacing animal testing?
A: While OOCs significantly reduce the reliance on animal testing, especially in early-stage drug discovery and toxicity screening, they are currently viewed as complementary tools. They excel in human-relevant mechanistic studies and toxicity prediction, but systemic effects, complex immune responses, and long-term interactions still necessitate some in vivo studies. The goal is to integrate OOCs to refine and reduce animal use.
Q: What cell types can be used in Organ-on-a-Chip devices?
A: A wide variety of cell types can be cultured in OOCs, including primary human cells, induced pluripotent stem cell (iPSC)-derived cells (which are particularly valuable for personalized medicine), immortalized cell lines, and even patient-derived cells. Co-culture of multiple cell types is often employed to better mimic tissue complexity.
References
-
Krysko D V, Demuynck R, Efimova I, et al. In vitro veritas: from 2D cultures to organ-on-a-chip models to study immunogenic cell death in the tumor microenvironment. Cells, 2022, 11(22): 3705. https://doi.org/10.3390/cells11223705
-
Koyilot M C, Natarajan P, Hunt C R, et al. Breakthroughs and applications of organ-on-a-chip technology. Cells, 2022, 11(11): 1828. https://doi.org/10.3390/cells11111828
-
Distributed under Open Access license CC BY 4.0, without modification.
Research Model
Related Sections:

 Figure 1 Two-dimensional models to study ICD.1,3
Figure 1 Two-dimensional models to study ICD.1,3
 Figure 2 Some of the advantages of OOAC technology over cell cultures and animal models.2,3
Figure 2 Some of the advantages of OOAC technology over cell cultures and animal models.2,3