B cell-based immunotherapy has shown great potential in cancer treatment applications. B cells in the mature tertiary lymphoid structures (TLS) could not only secrete antibodies but also mediate tumor apoptosis, phagocytosis, and antibody-dependent cellular cytotoxicity (as shown in Fig.1), which results in improving patient overall survival. To help overcome the obstacles of taking B-cell immunotherapy into practice, Creative Biolabs provides the in vivo survival assessment service to fulfill the related critical needs.
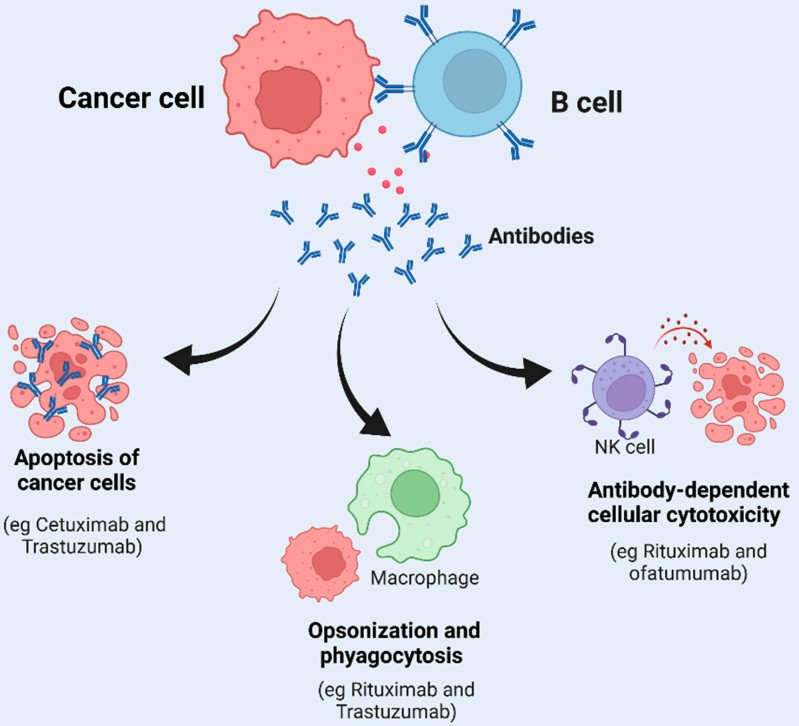 Fig.1 Key factors affecting B-cell function and tumor survival. (Gupta, et al., 2022)
Fig.1 Key factors affecting B-cell function and tumor survival. (Gupta, et al., 2022)
We offer a comprehensive service, from study design to experimental detail, to ensure high-quality research results and good customer service. For more information about the assessment of B cell-based immunotherapy, please inquire about our Engineered B Cells Assessment Service.
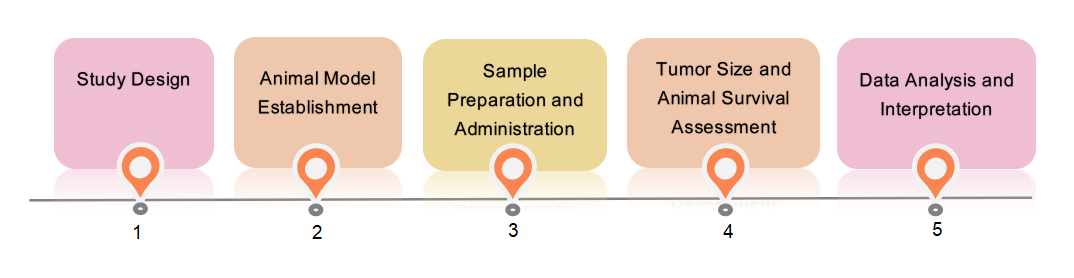 Fig.2 Our in vivo survival assessment service. (Creative Biolabs)
Fig.2 Our in vivo survival assessment service. (Creative Biolabs)
 Fig.3 Our features and benefits. (Creative Biolabs)
Fig.3 Our features and benefits. (Creative Biolabs)
Paper Title: CD40-Activated B Cell Cancer Vaccine Improves Second Clinical Remission and Survival in Privately Owned Dogs with Non-Hodgkin's Lymphoma
Technology: CD40-B cancer vaccination; in vivo survival assessment service
Animal model: Dogs with multicentric NHL
Journal: PLoS ONE
Published: 2011
Background: Cell-based active immunotherapy for cancer is a promising novel strategy. CD40-B may represent a viable cell-based vaccination strategy.
Results: The clinical and immunological results suggest that cell-based CD40 cancer vaccination is safe and synergizes with chemotherapy to improve clinical outcomes in canine NHL.
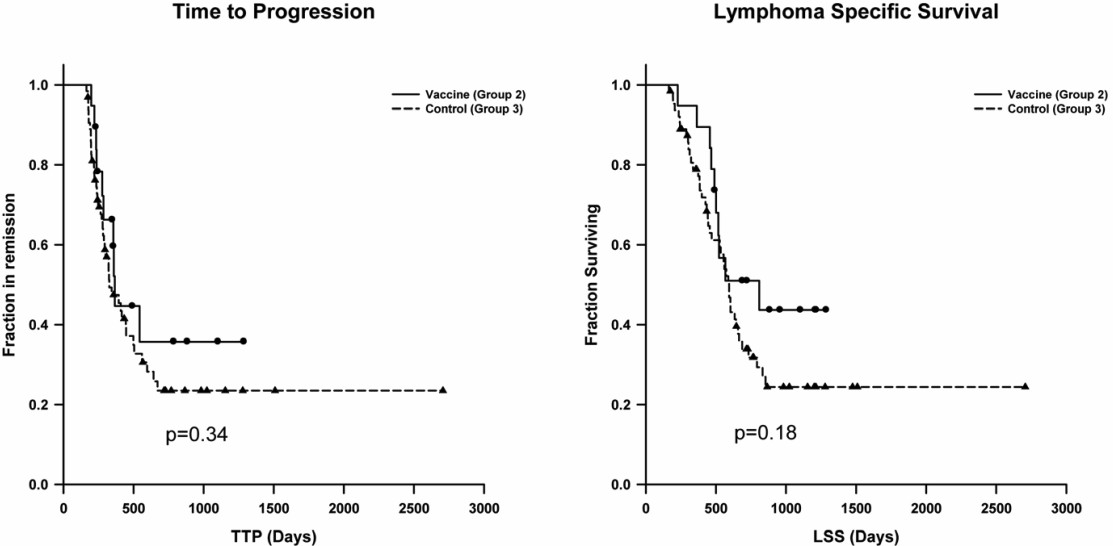 Fig.4 Comparison of vaccinated Group 2 to control Group 3 for time-to-progression (TTP) and lymphoma-specific survival (LSS). (Sorenmo, et al., 2011)
Fig.4 Comparison of vaccinated Group 2 to control Group 3 for time-to-progression (TTP) and lymphoma-specific survival (LSS). (Sorenmo, et al., 2011)
Q: Why there is a need to do the in vivo survival assessment?
A: After complicated modification, transplantation, and differentiation processes, we need to identify whether the B cells are safe, stable, homing to lymphatic tissues, and functioning well. The survival assessment is the key evidence to prove that B cell immunotherapy could improve tumor survival.
Fig.5 Our featured services. (Creative Biolabs)
Creative Biolabs provides the in vivo survival assessment with customizable design, standardized operation, and scientific analysis. We are always online to offer professional consultation on this assessment project, whenever you contact us for tailored information.