Creative Biolabs specializes in the development, validation, and profiling of biomarker assays, utilizing multiplex biomarker panels. We employ immunoanalytical and enzymatic platforms, as well as LC-MS/MS where appropriate. With extensive experience in custom multiplex designs, we can expertly assist in developing a biomarker assay tailored to your specific research requirements.
Promising Multiplex Immunoassays
Multiplex immunoassays represent a significant advancement over traditional singleplex methods, enabling the simultaneous detection and quantification of multiple analytes within a single sample. This innovative approach offers a comprehensive snapshot of complex biological systems, which is crucial for understanding disease mechanisms and treatment responses. Contemporary multiplex immunoassay systems primarily fall into two distinct formats: planar arrays and microbead-based suspension arrays.
- Planar assays involve immobilizing different capture antibodies at discrete locations on a solid surface, allowing for spatial separation of analytes.
- Microbead-based suspension arrays utilize distinct sets of color-coded microspheres, each conjugated with a specific capture antibody, which are then analyzed in suspension, offering high-throughput capabilities and flexible panel design.
Both formats leverage the specificity of antigen-antibody interactions combined with various detection methods to provide accurate quantitative readouts.
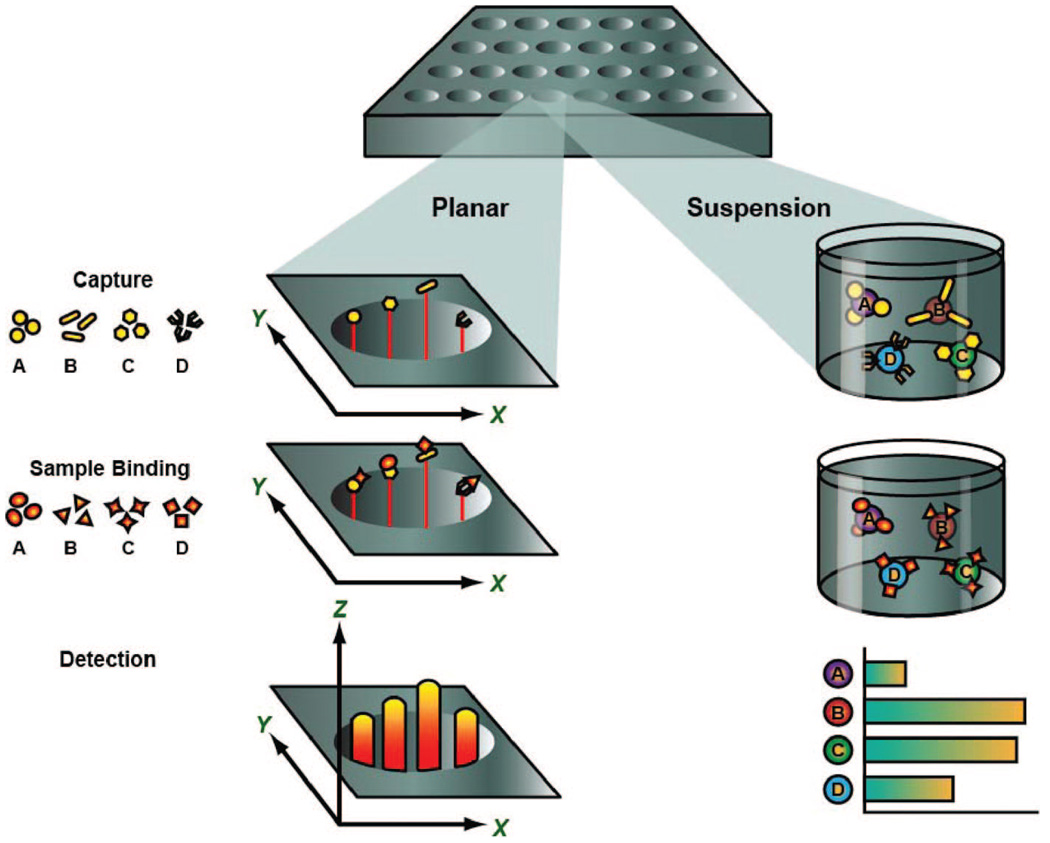 Fig.1 Multiplex formats in common use include planar-based assays or suspension-based assays.1
Fig.1 Multiplex formats in common use include planar-based assays or suspension-based assays.1
Advantages of Multiplex Immunoassays
- Optimized Sample Utilization: Multiplex immunoassays significantly reduce the required sample volume, allowing researchers to extract substantially more data from precious and limited biological specimens, thereby maximizing information yield.
- Enhanced Throughput and Efficiency: By simultaneously measuring multiple targets, these assays dramatically increase experimental throughput, leading to faster data acquisition and a more efficient allocation of research resources.
- Superior Data Quality: Standardized assay conditions across all analytes within a single reaction minimize inter-assay and intra-assay variability, resulting in highly reliable and reproducible quantitative data.
- Cost-Effective Solutions: Consolidating multiple individual tests into a single multiplex assay reduces the consumption of reagents, consumables, and labor, translating into significant cost savings for your research projects.
Our On-Demand Multiplex Assays Design and Development Services
At Creative Biolabs, our dedicated scientists are poised to assist you in designing and meticulously qualifying study-specific biomarker assays tailored to your precise requirements. While ELISA is traditionally a singleplex format, our expertise allows us to adapt this method for comprehensive multiplex protein analysis, combining monoclonal, polyclonal, or recombinant antibodies with defined specificity, sensitivity, and stability into a single test to facilitate the binding of multiple proteins. We offer seamless assay transfer and qualification for existing methods or can develop entirely new assays from conception to completion. Once an assay is rigorously developed and validated, we provide fast and accurate testing services for your preclinical and clinical samples, ensuring robust and reliable data.
Service Workflow
Our multiplex assay service workflow is meticulously designed for clarity and efficiency, ensuring a seamless experience from initial contact to comprehensive data delivery. Here's a step-by-step breakdown of how we partner with you:
The process begins with a detailed initial consultation where our expert team engages closely with you. We'll thoroughly discuss your specific research objectives, identify target analytes, and understand your sample types, enabling us to clearly define the precise scope and requirements of your project.
Following the consultation, we'll guide you on the starting materials required for your project. This typically involves providing your biological samples, such as serum, plasma, or tissue lysates, along with any specific reagents or antibodies you wish to incorporate into the assay.
Next, we proceed with the assay design and development phase. Our scientists meticulously optimize the antibody panels, refine assay conditions, and perfect detection methods to achieve optimal performance tailored precisely to your unique project's needs. This critical stage includes thorough internal validation steps to guarantee the robustness and reliability of the assay.
Once the assay is fully developed, qualified, and ready for use, we move to the sample analysis phase. Your submitted samples are processed using our validated multiplex platform, meticulously generating the quantitative data for your target biomarkers.
Upon completion of the analysis, the final delivery includes comprehensive data reports, detailed methodology, and expert interpretation of the results. This empowers you with actionable insights, facilitating your continued research and accelerating your path to groundbreaking discoveries.
Published Data
1. Multiplex Immunoassay Platform for the Simultaneous Detection of Serum IgG Antibodies Targeting Six Human Coronaviruses
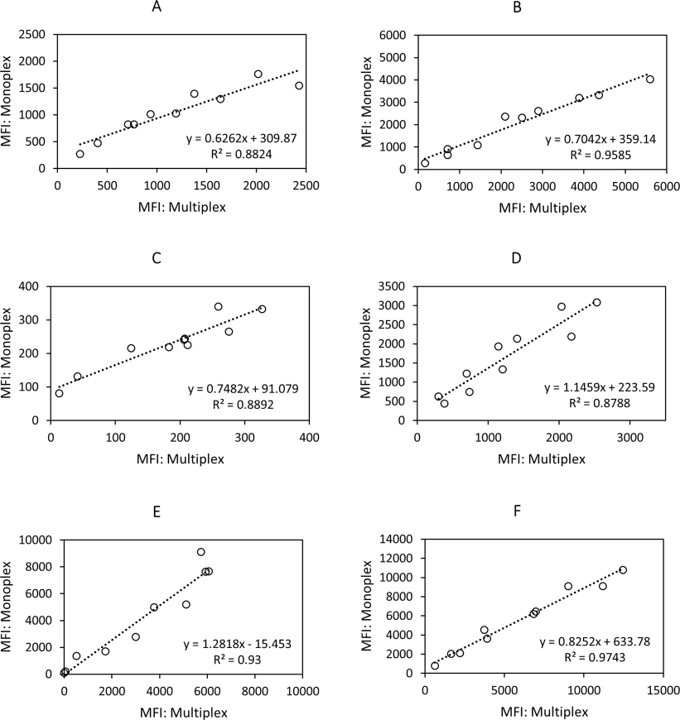 Fig.2 Correlation between monoplex and multiplex assay.2
Fig.2 Correlation between monoplex and multiplex assay.2
In this study, a novel multiplexed magnetic microsphere immunoassay (MMIA) platform was developed and evaluated, enabling simultaneous IgG detection against six human coronaviruses (hCoVs) recombinant nucleocapsid proteins. Researchers employed paired human serum samples to assess IgG reactivity against six hCoVs, evaluating the sensitivity, specificity, and reproducibility of the assay. The results showed no signal interference occurred between monoplex and multiplex formats, with R² values ranging from 0.87 to 0.97. MMIA detected IgG antibodies with 86% sensitivity (92 of 106 positive samples) and 84% specificity (68 of 80 negative samples). This study demonstrates the feasibility of using MMIA as a platform for conducting large-scale seroprevalence studies of hCoVs.
2. Development of Multiplex Microsphere Immunoassays for Arboviral IgM and IgG Detection
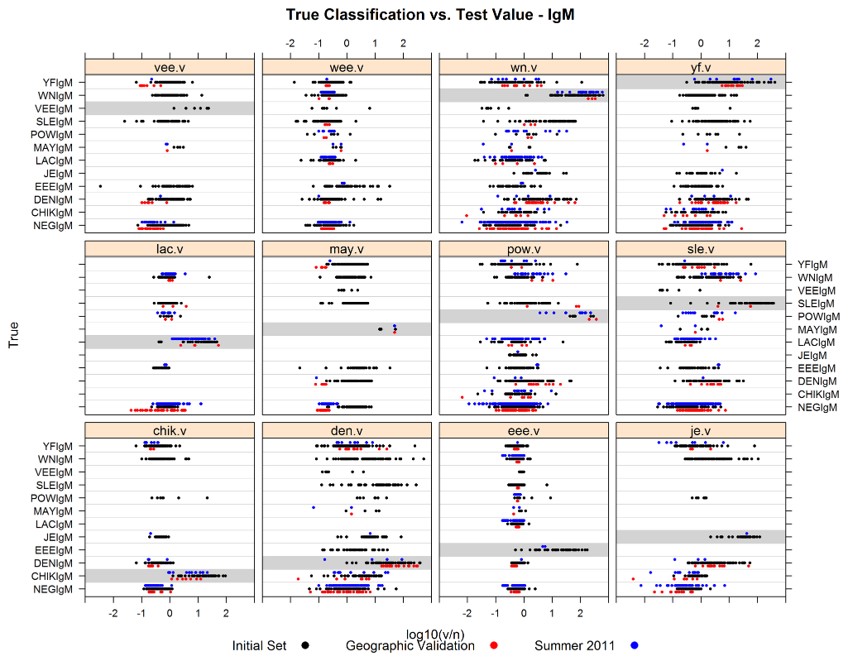 Fig.3 IgM based on median fluorescent intensity (MFI) of sample reacted on viral antigen/negative antigen.3
Fig.3 IgM based on median fluorescent intensity (MFI) of sample reacted on viral antigen/negative antigen.3
This study developed a 13-virus multiplexed IgM and IgG microsphere immunoassay (MIA) for arboviruses, incorporating both internal and external controls. Traditional serodiagnosis for arboviruses often involves a combination of individual ELISAs and plaque-reduction neutralization tests, which can be cumbersome, especially when testing multiple viruses from specific geographic regions. The new multiplex assay allowed concurrent testing for multiple viruses, improving efficiency. The study evaluated 8 statistical classification methods to determine the best approach for analyzing results, with a focus on geographic regions. The "Logitboost" logistic regression method was identified as the most efficient for classification. When combining data from all tested samples, the error rates for the multiplex IgM and IgG MIAs were less than 5% for all geographic batteries. This work represents the most comprehensive, validated multiplexing method for arboviruses and offers a systematic approach to selecting optimal classification strategies for serologic testing.
Service Highlights
- Customization and Flexibility: Our services are meticulously designed to provide highly customized multiplex panel configurations, ensuring precise alignment with your unique research objectives and accommodating diverse sample types.
- Rigorous Validation Protocols: Every assay we develop or qualify undergoes stringent fit-for-purpose biomarker validation, guaranteeing the accuracy, precision, and reliability of your results for confident interpretation.
- Advanced Technological Platforms: We leverage a diverse array of cutting-edge technologies, including bead-based immunoassays, ECL, mIHC/mIF, and MS-based approaches, to deliver superior performance and comprehensive insights.
- End-to-End Scientific Support: From initial consultation and assay design to data analysis and interpretation, our team of seasoned biologists provides unparalleled scientific expertise, acting as your dedicated partner throughout the entire process.
FAQs
-
How do you ensure the accuracy of multiplex biomarker assay results?
We ensure the accuracy of multiplex biomarker assay results by implementing rigorous fit-for-purpose validation protocols. These protocols involve extensive testing of bioanalytical parameters, including accuracy, precision, and sensitivity, to guarantee that the measurements closely reflect the true concentrations of the analytes in the samples.
-
Can I integrate my existing antibodies into Creative Biolabs' custom multiplex assay designs?
Yes, you can integrate your existing antibodies into Creative Biolabs' custom multiplex assay designs. Our expert scientists will work collaboratively with you to assess the compatibility and performance of your specific monoclonal, polyclonal, or recombinant antibodies within our multiplex assay platforms.
-
How does multiplexing reduce experimental costs compared to singleplex assays?
Multiplexing reduces experimental costs by consolidating multiple individual tests into a single assay, thereby decreasing the consumption of reagents, consumables, and labor. This streamlined approach minimizes overheads and optimizes resource utilization across the entire experimental process.
-
Do you offer services for both protein and nucleic acid biomarker profiling?
We primarily focus on protein biomarker profiling through our immunoassay and mass spectrometry platforms. While our core expertise lies in protein analysis, we can discuss potential collaborations or guide you to resources for nucleic acid profiling if your project requires it.
-
How does Creative Biolabs handle data analysis and interpretation for complex multiplex panels?
Creative Biolabs handles data analysis and interpretation for complex multiplex panels by employing advanced bioinformatic tools and our team's deep scientific expertise. We provide comprehensive reports that include raw data, statistical analysis, and expert interpretation, helping you derive meaningful biological insights from your results.
-
Is Creative Biolabs equipped to handle large-scale biomarker screening projects?
Yes, Creative Biolabs is well-equipped to handle large-scale biomarker screening projects. Our state-of-the-art platforms and streamlined workflows are designed to deliver high-throughput capabilities, making us an ideal partner for extensive biomarker discovery and validation initiatives.
Our biomarker platform offers several complementary solutions to fit every need you may have in terms of protein quantitation and profiling, as well as phosphorylation or glycosylation profiling, and mRNA/miRNA profiling. If you are interested in our multiple biomarker analysis services, please feel free to contact us for more information.
References
- Ahsan, Haseeb, and Rizwan Ahmad. "Multiplex technology for biomarker immunoassays." Innate Immunity in Health and Disease (2020): 1-10.
- Trivedi, Suvang U., et al. "Development and evaluation of a multiplexed immunoassay for simultaneous detection of serum IgG antibodies to six human coronaviruses." Scientific reports 9.1 (2019): 1390. Distributed under Open Access license CC BY 4.0, without modification.
- Basile, Alison J., et al. "Multiplex microsphere immunoassays for the detection of IgM and IgG to arboviral diseases." PloS one 8.9 (2013): e75670. Distributed under Open Access license CC0 1.0.
For Research Use Only.