Microfluidic technology provides numerous advantages over the traditional macroscale methods and slowly emerges as new platforms in protein detection in the in vitro diagnostic (IVD) area. Empowered by an elite team of experienced scientists, Creative Biolabs provides the best strategy and protocols customized to support in vitro protein diagnosis by Microfluidic chips for disease research.
Current Detection Methods and Status
Gene detection has opened up a few applications in disease diagnosis, but challenges remain throughout the entire analytical process, from the collection and treatment of specimens to the amplification and detection of nucleic acid. While DNA or RNA analysis can provide important diagnostic information, protein analysis is particularly valuable because proteins are more direct mediators of normal and diseased cellular processes, thus offering many opportunities for diagnosing, stratifying, and monitoring disease. Other techniques like immunoassays (ELISA) have been used to detect biomarkers, however, they can be time-consuming, expensive and are mostly carried out in a laboratory requiring skilled personnel. In most cases, immunoassays look for only one biomarker and are not sensitive enough to detect very low biomarker levels especially at the early stages of the disease.
Integration of Protein Detection and Microfluidics
To be well suited for IVD application, a proteomic technology must be sensitive enough to detect endogenous levels of low abundance proteins, require low sample volumes, have a short assay time, and ideally be portable as to allow informed treatment decisions to be made at the point of care (POC). Microfluidics, with its inherent advantages of low sample and reagent volumes, precise control over the microenvironment, multiplex and automated capabilities, has emerged as a promising operation platform with which to develop sensitive proteomic technologies.
Application Examples
In the reported study, scientists developed a novel proteomic platform that can easily be translated into a protein biomarker diagnostic. This platform integrates Microfluidic technology with electrical impedance sensing and embodies a unique two-chamber architecture in which the capture/reaction and detection steps are physically separated from one another. This two-chamber design offers the potential for greater sensitivity as the capture/reaction and detection steps can be independently optimized. The implementation and feasibility of this platform have been demonstrated by determining the protein abundance and activity of IL-6 and AbI kinase.
An example of a Microfluidic detector using antibodies against viral proteins was described. Scientists developed a device capable of recognizing two subtypes of Influenza A simultaneously based on antibodies immobilized on magnetic beads. Antibodies anti-H7 and anti-H9 were used to detect H7N9 and H9N2 viral proteins in concentrations as low as 3.4 ng/mL and 4.5 ng/mL, respectively. Another way to detect infections, instead of directly looking for the pathogenic agent, is to detect the presence of those antibodies already present in the sample. Besides, integrated Microfluidics paved the way for protein modification analysis. For instance, autophosphorylation is a biochemical process associated with a great variety of normal and pathological processes ranging from cancer to complex developmental disorders.
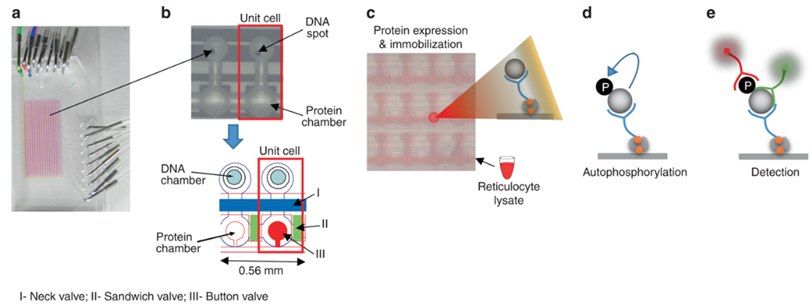 Fig.1 High-throughput integrated Microfluidics strategy for tyrosine autophosphorylation discovery.1, 2
Fig.1 High-throughput integrated Microfluidics strategy for tyrosine autophosphorylation discovery.1, 2
Featured Services at Creative Biolabs
According to the properties of the protein to be tested and the requirements for signal amplification, Creative Biolabs will develop innovative assay and Microfluidic chips to remove the obstacles of your projects and bring breakthroughs in IVD. Most of our diagnostic mechanisms for protein biomarker involve two steps, an initial immunoassay to capture the protein analyte and subsequent detection of the captured analyte. Fluorescence, chemiluminescence, colorimetric methods, electrochemistry-based detectors are available detection methods used in our Microfluidic systems. Besides, our protein biomarker analysis focuses on the interface of Microfluidics with immunohistochemistry (IHC), ELISA, and mass spectrum (MS). Currently, our services are mainly focused on offering protein detection by Microfluidic chips for disease diagnosis, including but not limited to:
- Protein modification research
- Protein identification and quantitation
- Microfluidic chip development
- Microfluidic-based POCT Development
If you are interested in our integrated protein detection services on the Microfluidic chip, please feel free to contact us for more information.
Published Data
1. A Rapid Microfluidic Immuno-Biosensor Platform for the Point-of-Care Determination of Urinary High-Sensitivity CRP
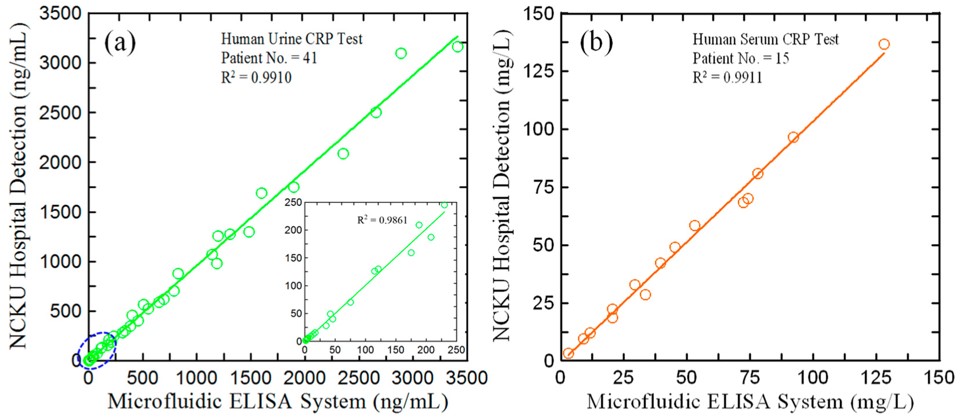 Fig.2 Comparison of CRP detection results obtained by PETIA and microfluidic system.3,2
Fig.2 Comparison of CRP detection results obtained by PETIA and microfluidic system.3,2
This study introduced a microfluidic ELISA detection system for quantifying high-sensitivity C-reactive protein (hs-CRP) in urine. The process used a specialized ELISA method, where capture and detection antibodies were pre-coated onto the microchip substrate, forming an immune complex with the target antigen. Horseradish peroxidase (HRP) was added as a marker enzyme, and a colorimetric reaction was triggered using 3,3′,5,5′-tetramethylbenzidine. The resulting absorbance values were measured by a micro-spectrometer and used to determine hs-CRP concentration based on a calibration curve. The assay took 50 minutes to complete and demonstrates recovery rates of 93.8–106.2% in blind water samples and 94.5–104.6% in artificial urine. The detection results for 41 urine samples from chronic kidney disease patients were highly consistent with those obtained using the conventional homogeneous PETIA method (R² = 0.9910). This microfluidic system proved effective for CRP detection in both urine and serum, providing an accurate and reliable tool for point-of-care monitoring of hs-CRP concentrations.
2. A Rapid Microfluidic Quaking-Induced Conversion Assay for On-Site Amplification and Visual Detection of Misfolded Proteins
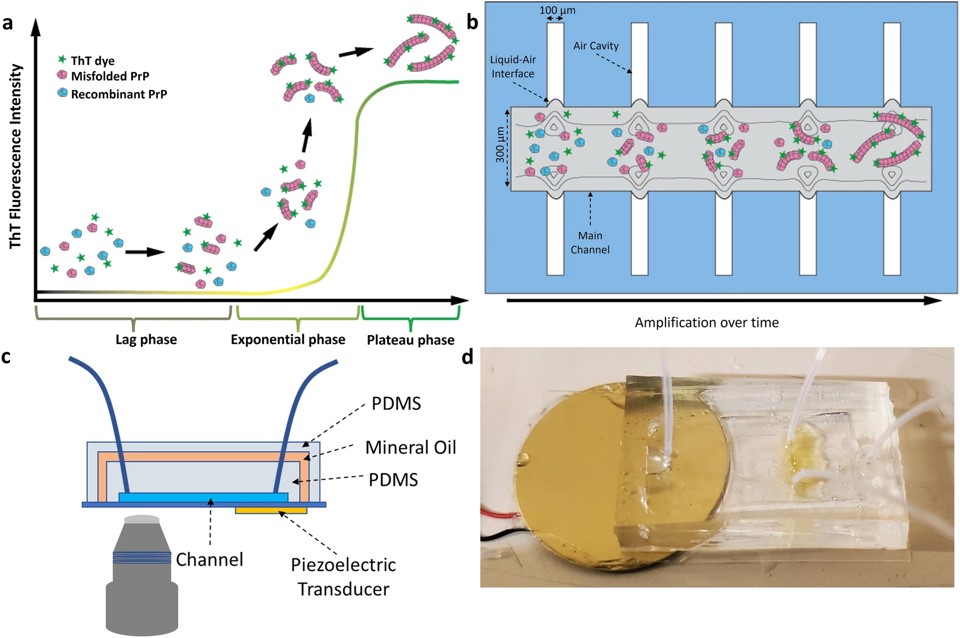 Fig.3 Overview of the Micro-QuIC assay.4,2
Fig.3 Overview of the Micro-QuIC assay.4,2
This study presented an acoustofluidic platform, microfluidic quaking-induced conversion (Micro-QuIC), designed for the fast, precise detection of protein misfolding diseases. Using chronic wasting disease (CWD) in wild white-tailed deer as a model, Micro-QuIC notably accelerated prion amplification compared to the gold-standard real-time quaking-induced conversion (RT-QuIC) method. The system utilized acoustofluidic mixing for efficient reagent mixing, reducing turnaround time. Additionally, Micro-QuIC combined a gold nanoparticle-based detection technique visible to the naked eye, allowing for visual differentiation between CWD-positive and CWD-negative samples. This creates a fast, point-of-care platform for detecting misfolded proteins in various diseases.
References
- Nevenzal, Hadas, et al. "A high-throughput integrated microfluidics method enables tyrosine autophosphorylation discovery." Communications Biology 2.1 (2019): 42.
- Distributed under Open Access license CC BY 4.0, without modification.
- Chen, Szu-Jui, et al. "Rapid Microfluidic Immuno-Biosensor Detection System for the Point-of-Care Determination of High-Sensitivity Urinary C-Reactive Protein." Biosensors 14.6 (2024): 283.
- Lee, Dong Jun, et al. "Rapid on-site amplification and visual detection of misfolded proteins via microfluidic quaking-induced conversion (Micro-QuIC)." npj Biosensing 1.1 (2024): 6.
For Research Use Only.