The impact of the rapid expansion of single cell analysis based on Microfluidics is evident in its great potential for numerous applications, including drug discovery, diagnostics, cancer research, regenerative medicine, system and synthetic biology, and many others. With our highly experienced staff, Creative Biolabs is confident in offering the best single cell analysis services based on Microfluidics to guarantee the most satisfactory results for our customers worldwide.
Single Cell Analysis based on Microfluidics
Microfluidics-platform based on microwells, microvalves, and droplets has emerged as critical enabling tools for high-throughput analysis and single cells sorting in recent years. The implementation of Microfluidic technologies in single cell analysis offers rich information and high throughput screening. It enables the creation of innovative conditions that are impractical or impossible by conventional means. Microfluidics enables the encapsulation and activation of single cells and also allows confining soluble metabolites secreted by activated cells in a relatively small volume. Compartmentalization of single cells in droplets enables the analysis of proteins released from or secreted by cells and links genotype with phenotype through compart-mentalization, thereby overcoming one of the significant limitations of traditional assays. Besides, Microfluidic techniques allow for precise control of the localized microenvironment, which yields more accurate outcomes.
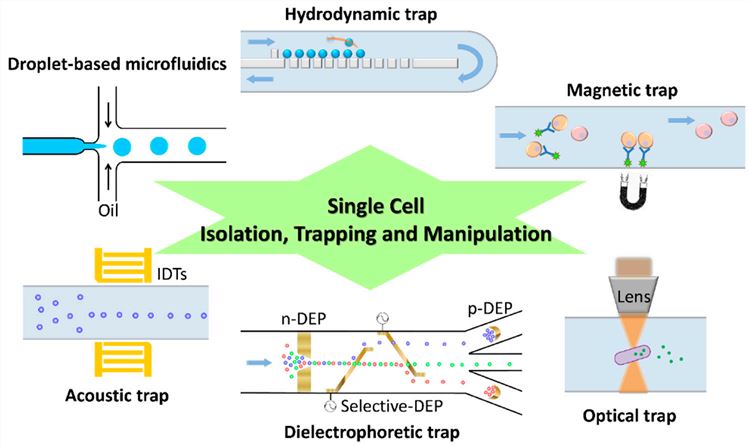 Fig.1 Illustrative representation of various methodologies for the capture, isolation, and handling of individual cells within a microfluidic platform.1, 2
Fig.1 Illustrative representation of various methodologies for the capture, isolation, and handling of individual cells within a microfluidic platform.1, 2
Our Single Cell Analysis Services based on Microfluidics
Recent advances in Microfluidics enable systematic high-throughput analyses of individual cells in a highly controlled manner, which has revolutionized both fundamental and applied research fields. By providing high-quality and customized solutions and services, Creative Biolabs is a reliable partner for your single cell analysis development based on Microfluidic technology. Various types of analysis combined with Microfluidic systems for single cell analysis are available at Creative Biolabs, including but not limited to:
- Single living cell detection
- Cell lysate analysis
- Single cell sequencing
- Single cell genomics
- Single cell epigenomics
- Single cell transcriptomics
- Single cell proteomics
- Single cell metabolomics
- Enzyme analysis of single cells
- Functional studies on single cells
- Drug studies on single cells
- Single cell immunology
The ease of generation combined with a simple encapsulation of single cells using a dilute suspension makes Microfluidic the ideal high-throughput platform for single cell analysis. Creative Biolabs is a dependable service provider to offer custom single cell analysis services based on advanced Microfluidic technology for our global customers. Please do not hesitate to contact us with your particular needs.
Published Data
1. Drop-Based Microfluidic Platform for High-Throughput Single-Cell Transcriptomics of RNA-Seq
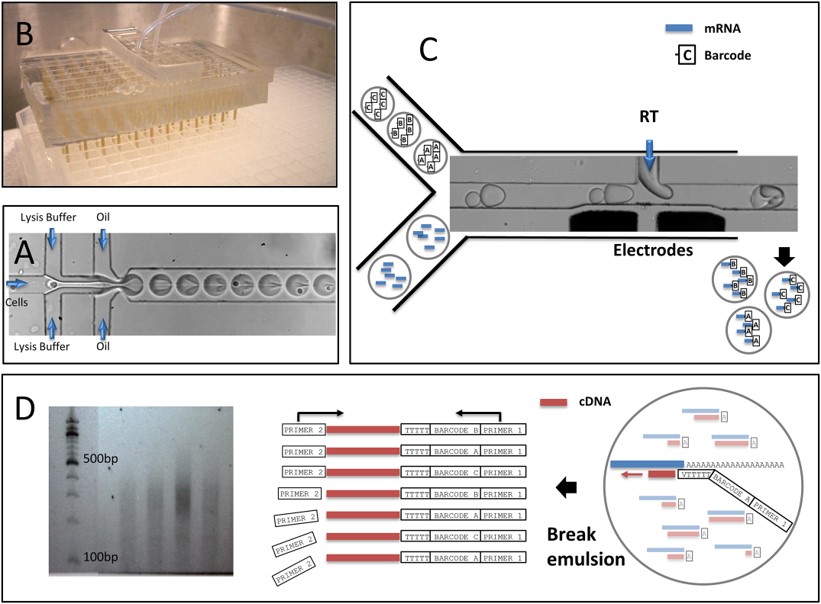 Fig.2 Experimental workflow of Hi-SCL RNA-Seq.3,2
Fig.2 Experimental workflow of Hi-SCL RNA-Seq.3,2
This paper introduced high-throughput single-cell labeling (Hi-SCL), a technique that utilized drop-based libraries of oligonucleotide barcodes to uniquely label and index individual cells within a population. The method was highly scalable by using drops as containers and a microfluidics platform to handle them in large quantities. Labeled molecules from different cells could be combined while preserving the information about their original cell sources. The method was demonstrated for producing RNA sequencing data from a variety of individual cells. Barcoded oligonucleotides prime cDNA synthesis within drops, and the barcoded cDNAs were sequenced and deconvoluted to obtain single-cell mRNA expression data. Proof-of-concept experiments showed that Hi-SCL provided comparable data to existing methods, with the potential for high-throughput analysis of large cell populations.
2. Microfluidic Chip for Single-Cell Trapping and Signaling Dynamics Analysis
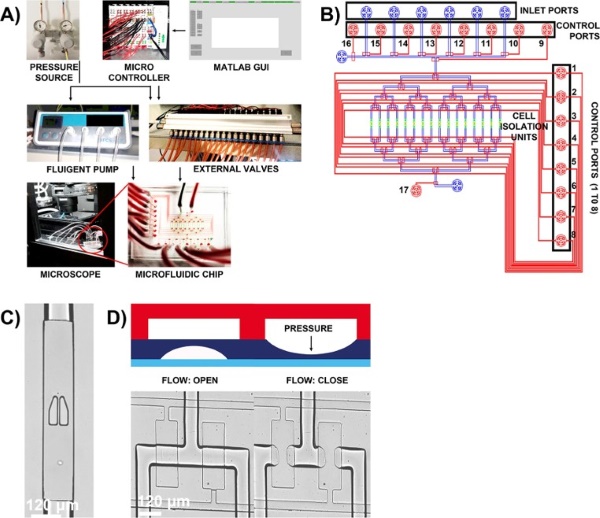 Fig.3 Schematic of two-layer microfluidic design.4,2
Fig.3 Schematic of two-layer microfluidic design.4,2
This study presented a fully automated, integrated microfluidic chip for delivering input signals to single, isolated suspension or adherent cells with precise control. Researchers used the system to analyze different cell types, observing real-time dynamics of caspase 3 activation during DMSO-induced apoptosis in K562 cancer cells and STAT-1 translocation triggered by interferon γ (IFNγ) in NIH3T3 fibroblasts. The findings demonstrate the microfluidic system's potential for investigating temporal single-cell signaling networks, where changes in outputs reveal signal processing mechanisms.
References
- Lo, Shih-Jie, and Da-Jeng Yao. "Get to understand more from single-cells: current studies of microfluidic-based techniques for single-cell analysis." International journal of molecular sciences 16.8 (2015): 16763-16777.
- Distributed under Open Access license CC BY 4.0, without modification.
- Rotem, Assaf, et al. "High-throughput single-cell labeling (Hi-SCL) for RNA-Seq using drop-based microfluidics." PloS one 10.5 (2015): e0116328.
- Sinha, Nidhi, et al. "Microfluidic chip for precise trapping of single cells and temporal analysis of signaling dynamics." Communications Engineering 1.1 (2022): 18.
For Research Use Only.