Lactobacillus paracasei-derived Exosome Research & Application
Overview Workflow Bioactive Insights Support Platform Why Us Testimonials FAQs
Introduction and Background
Lactobacillus paracasei, a well-characterized member of the lactic acid bacteria family, is widely distributed in fermented foods and in the gastrointestinal tract of humans. Over the past decade, scientific attention has moved beyond the metabolic properties of this probiotic species toward the extracellular vesicles (EVs) and exosome-like structures it secretes. These nanosized vesicles are now recognized as mediators of microbial communication, carrying proteins, lipids, nucleic acids, and other molecules that influence host biology.
Growing evidence suggests that L. paracasei-derived exosomes may regulate immune responses, modulate inflammatory signaling pathways, and even affect tumor biology. Their small size and ability to interact with host cells make them an emerging subject of basic microbiology, immunology, and food science. While much remains to be uncovered, the study of L. paracasei vesicles has already broadened our understanding of probiotic functionality.
At Creative Biolabs, we specialize in providing tailored services for Gram-positive bacterial exosome research, including customized projects involving L. paracasei. Our aim is to support academic groups and industrial laboratories in deciphering the complex biology of these vesicles through reliable workflows and a flexible service platform.
Streamlined Exosome Preparation Workflow
The process of isolating vesicles from L. paracasei involves a combination of culture, separation, and concentration steps designed to enrich for exosome-like structures while minimizing contaminants. Creative Biolabs follows a custom-development strategy, where the fundamental workflow focuses on isolation, and additional downstream characterization can be provided if requested.
Standard Workflow (Core Development Service)
-
Culture Initiation – L. paracasei is grown in optimized broth conditions at 37 °C under controlled agitation to achieve high-density growth.
-
Supernatant Collection – Cells are separated from the liquid culture by centrifugation, leaving a cell-free medium rich in secreted vesicles.
-
Initial Filtration – Residual bacterial cells and large debris are removed using filtration membranes.
-
Concentration & Ultracentrifugation – Vesicles are enriched by ultrafiltration and serial high-speed centrifugation steps to pellet nanosized fractions.
-
Vesicle Recovery – The precipitated exosomes are resuspended in sterile PBS or equivalent buffer and stored under controlled frozen conditions.
Optional Add-On Services
Depending on project needs and availability of reference libraries for L. paracasei, Creative Biolabs can provide additional characterization steps (optional):
-
Proteomic profiling (mass spectrometry-based analysis of vesicle cargo proteins).
-
Nucleic acid content analysis (RNA/DNA carried within vesicles).
-
Biophysical characterization (size distribution, zeta potential, morphology).
These optional modules allow research teams to expand from isolation into a deeper functional or mechanistic exploration while ensuring that the primary workflow remains standardized and reproducible.
Functional Insights: Bioactive Features of L. paracasei Vesicles
Scientific studies on L. paracasei-derived exosomes have reported several intriguing findings. Below is a synthesized overview of the research progress, presented in table format for clarity. Note that these findings are results from independent academic studies, not proprietary discoveries of Creative Biolabs.
|
Research Focus
|
Key Observations
|
|
Regulation of immune activity in macrophages and colon cells
|
Pretreatment with vesicles suppressed lipopolysaccharide-induced NO and TNF-α in macrophages; in colon epithelial cells, exosomes reduced pro-inflammatory cytokines while increasing anti-inflammatory mediators.
|
|
Biodistribution after oral administration
|
In vivo imaging showed vesicles initially localized in the stomach, later concentrated in the colon (24 h), and cleared by 48 h, suggesting time-dependent distribution dynamics in the GI tract.
|
|
Impact on colitis models
|
Mice exposed to both lipopolysaccharide and vesicles exhibited improved survival, less weight loss, and reduced pathology. Inflammatory markers such as COX-2 and IL-1β were downregulated.
|
|
Mechanistic role of ER stress
|
Western blot analyses indicated vesicle-mediated activation of ER stress pathways. The anti-inflammatory effect was linked to CHOP upregulation, which was diminished when ER stress was pharmacologically blocked.
|
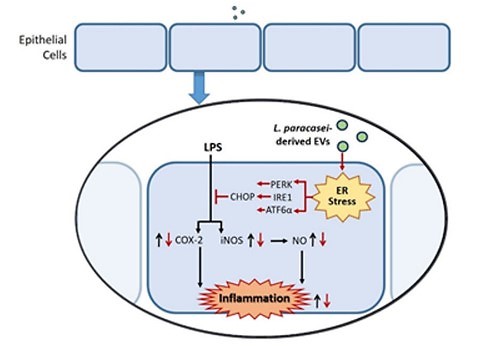 Fig.1 Mechanistic insights into colitis suppression via exosomes derived from Lactobacillus paracasei.1
Fig.1 Mechanistic insights into colitis suppression via exosomes derived from Lactobacillus paracasei.1
|
Research Focus
|
Key Observations
|
|
Effect on colon cancer cells
|
Exosomes inhibited invasion and promoted apoptosis of colon cancer cells in vitro; tumor xenografts in mice receiving vesicles displayed slower growth.
|
|
Molecular mechanisms of tumor inhibition
|
Multi-omics approaches (transcriptomics, western blotting, immunohistochemistry) demonstrated suppression of the PDK1/AKT/Bcl-2 signaling axis, suggesting a mechanistic basis for anti-tumor activity.
|
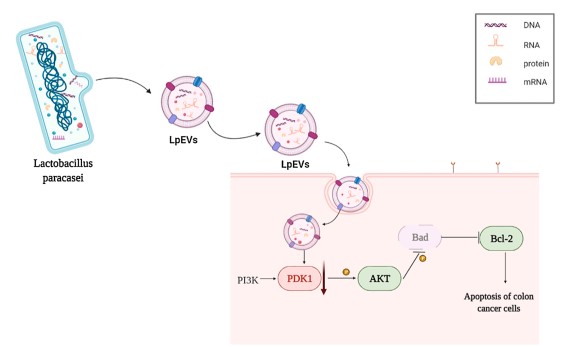 Fig.2 Molecular pathways through which Lactobacillus paracasei exosomes combat colon cancer.2
Fig.2 Molecular pathways through which Lactobacillus paracasei exosomes combat colon cancer.2
Collectively, these results underscore the bioactive capabilities of L. paracasei exosomes in influencing inflammation and altering cellular pathways. From a fundamental research standpoint, these findings prompt additional exploration into the cross-kingdom interactions facilitated by vesicles derived from probiotics.
Creative Biolabs' Comprehensive Research Support Platform
Our services extend from isolation to custom assays, ensuring that research teams have access to reliable resources at every stage of their projects.
Core Capabilities:
-
Customizable vesicle isolation services.
-
Access to reference data for Gram-positive bacteria (where available).
-
Optional add-ons including proteomics, RNA analysis, and functional assays.
-
Consultation for project design and experimental troubleshooting.
Collaborative Approach:
We recognize that each project is unique. Creative Biolabs supports scientists in tailoring experiments to specific questions, whether they relate to fundamental microbiology, host–microbe interactions, or probiotic biology. Our goal is to act as a dependable partner rather than a one-size-fits-all service provider.
Why Work with Creative Biolabs?

Technical Precision
Our workflows are rigorously standardized, ensuring reproducibility while allowing for customization.

Flexibility
Optional services can be layered onto the basic workflow, providing researchers with data breadth without unnecessary costs.

Deep Knowledge of Gram-Positive Systems
Creative Biolabs has developed significant expertise in managing Gram-positive vesicles, which present greater technical challenges compared to Gram-negative vesicles due to their distinct cell wall structures.

Responsive Support
Dedicated scientific teams are available to provide guidance, whether in project design, protocol optimization, or data interpretation.
Testimonials from Collaborating Scientists
"Working with Creative Biolabs allowed our team to efficiently isolate vesicles from probiotic strains we had never studied before. The clarity of their workflow and the transparency regarding optional services made the collaboration smooth and effective."
– Researcher, University Lab
" We were highly impressed by the technical expertise demonstrated by the staff at Creative Biolabs. Their ability to explain the limitations and possibilities of Gram-positive vesicle research helped us design more robust experiments."
– Group Leader, Biotechnology Institute
Lactobacillus paracasei-derived exosomes are an emerging research frontier that integrates microbiology, immunology, and food science. Their reported effects on inflammatory signaling and tumor biology highlight the need for continued systematic investigation. By providing organized workflows, customizable optional services, and expert assistance, Creative Biolabs enables researchers to conduct these studies with assurance. Creative Biolabs is dedicated to enhancing the understanding of Gram-positive bacterial vesicles. For laboratories interested in exploring L. paracasei-derived exosomes, our specialist team offers not just services, but a dependable partnership in discovery.
FAQs
Q: What are the key functions of Lactobacillus paracasei-derived exosomes?
A: Lactobacillus paracasei-derived exosomes play several crucial roles in microbial interactions and host communication. They are known to carry bioactive molecules that can modulate the immune response, enhance barrier functions in epithelial cells, and positively influence gut microbiota composition. Their ability to facilitate intercellular communication makes them essential for maintaining gut homeostasis.
Q: How do exosomes from Lactobacillus paracasei differ from exosomes derived from other probiotics?
A: Lactobacillus paracasei-derived exosomes exhibit specific molecular compositions that are tailored to their function within the gastrointestinal environment. These differences may include unique surface markers, distinct lipid profiles, and specific sets of RNA molecules that may enhance their ability to interact with host cells and other microorganisms. Ongoing research continues to elucidate these distinctions and their implications for probiotic efficacy.
Q: What is the potential application of Lactobacillus paracasei exosomes in food science?
A: In food science, Lactobacillus paracasei exosomes have the potential to act as natural delivery vehicles for functional compounds, enhancing the nutritional profile of fermented foods. They may also contribute to the development of novel functional foods by providing active ingredients that promote gut health and overall wellness, as well as serving as natural preservatives by possessing antimicrobial properties.
Q: Are there any notable findings regarding the role of Lactobacillus paracasei exosomes in modulating gut microbiota?
A: Lactobacillus paracasei exosomes can have a positive impact on the gut microbiota's composition by encouraging the growth of good bacteria and suppressing harmful strains. This modulation appears to be mediated through the delivery of specific molecules that promote microbial community balance and enhance the overall health of the gut ecosystem.
Q: How might Lactobacillus paracasei exosomes contribute to research on gut-brain axis communication?
A: Exosomes of Lactobacillus paracasei may be important in the gut-brain axis because they help the gut microbiota and central nervous system communicate. The bioactive components carried by these exosomes could influence neuroinflammatory responses and contribute to the synthesis of neurotransmitters, potentially impacting mood and cognitive functions. Ongoing research is focusing on these connections to uncover their implications for mental health.
Q: What are the potential avenues for further investigation into exosomes generated from Lactobacillus paracasei?
A: Future research will likely focus on further characterizing the molecular components of Lactobacillus paracasei-derived exosomes and elucidating their specific mechanisms of action. Additionally, exploring their potential in diverse applications such as biopreservation, functional food development, and targeted delivery systems for bioactive compounds will be key areas of investigation.
References
-
Choi, Ji Hyun, et al. "Lactobacillus paracasei-derived extracellular vesicles attenuate the intestinal inflammatory response by augmenting the endoplasmic reticulum stress pathway." Experimental & Molecular Medicine 52.3 (2020): 423-437. Distributed under Open Access license CC BY 4.0. The image was modified by extracting and using only Part e of the original image and revising the title. https://doi.org/10.1038/s12276-019-0359-3
-
Shi, Yangqian et al. "Extracellular vesicles of Lacticaseibacillus paracasei PC-H1 induce colorectal cancer cells apoptosis via PDK1/AKT/Bcl-2 signaling pathway." Microbiological research vol. 255 (2022): 126921. Distributed under Open Access license CC BY 4.0. The image was modified by revising the title. https://doi.org/10.1016/j.micres.2021.126921
For Research Use Only. Cannot be used by patients.
Related Services:

 Fig.1 Mechanistic insights into colitis suppression via exosomes derived from Lactobacillus paracasei.1
Fig.1 Mechanistic insights into colitis suppression via exosomes derived from Lactobacillus paracasei.1
 Fig.2 Molecular pathways through which Lactobacillus paracasei exosomes combat colon cancer.2
Fig.2 Molecular pathways through which Lactobacillus paracasei exosomes combat colon cancer.2













