Overview of DNA Vaccines
DNA vaccine refers to the direct injection of a recombinant eukaryotic expression vector encoding a certain protein antigen into the animal body, so that the foreign gene is expressed in vivo, and the antigen produced activates the immune system of the body, thereby inducing specific humoral immunity and cellular immunity. immune response. Although conventional vaccines are crucial for preventing the spread of numerous highly infectious diseases, the manufacturing of these vaccines often requires that researchers handle live pathogens. Compared to conventional protein/peptide-based vaccines intended to induce antigen-specific adaptive immune responses, DNA vaccines are more stable, cost-efficient, easy to manufacture, and safe in handling. The high number of DNA vaccines tested in clinical trials emphasizes their important role in future medical approaches. DNA vaccines are being investigated for various applications including therapy of cancer, allergies, autoimmune, and infectious diseases.
How do DNA vaccines work?
Like any other type of vaccine, DNA vaccines induce an adaptive immune response. DNA vaccines work by injecting genetically engineered plasmid containing the DNA sequence encoding the antigen(s) against which an immune response is sought, so the cells directly produce the antigen, thus causing a protective immunological response.
The classical ways for vaccine delivery are intramuscular, intradermal, and subcutaneous injections which address primarily myocytes and keratinocytes, respectively, but also antigen-presenting cells (APCs) residing near the injection side. In the case of DNA vaccines, after their internalization the DNA needs to translocate to the nucleus for transcription, followed by a translation in the cytoplasm. APC can be transfected directly by DNA vaccines. In this case, the encoded antigen is expressed, and after processing antigen-derived peptides are loaded in parallel onto MHC I and MHC II molecules. In case of concomitant activation, antigen-presenting APC migrates into the draining lymph node and can prime CD8+ and CD4+ T helper cells. In the case of cutaneous application, transfected keratinocytes may generate antigen which is released by exosomes or apoptotic bodies and is internalized by APC. In general, antigens of exogenous origin are loaded rather exclusively on MHC II resulting in the activation of helper CD4+ T cells which in turn contribute to B cell priming to yield a humoral immune response and are required for full activation of CD8+ T cells. After intramuscular application, transfected myocytes may undergo apoptosis. Apoptotic bodies are engulfed by APC and the exogenous antigen is cross-presented on MHC I resulting in a CD8+ T cell response. Early studies on DNA vaccination demonstrated that priming of CD8+ cytotoxic T lymphocytes (CTL) is mostly dependent on bone marrow-derived dendritic cells (DC) rather than induced by tissue-specific cells, and was strictly dependent on CD4+ T cell help. The most serious challenges for DNA vaccines intended to induce an anti-tumor immune response are caused by the immune evasion strategies of the tumor. In this regard, tumor cells are often characterized by defective processing of antigens for subsequent presentation via MHC I and impaired expression of MHC I.
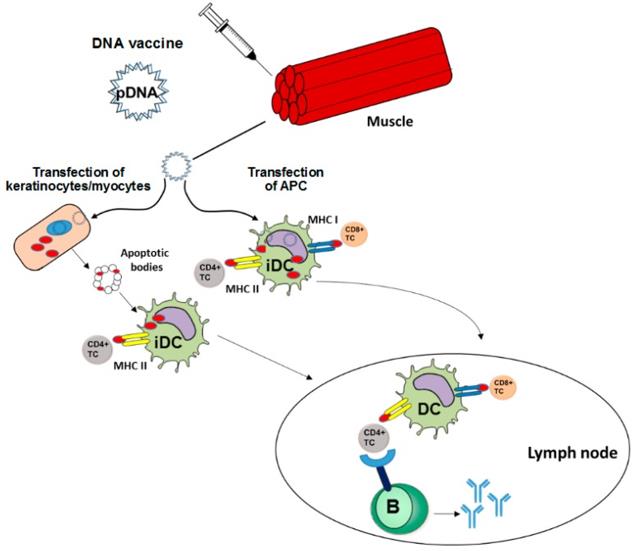 Figure 1 DNA Vaccines Induce Adaptive Immune Responses (Hobernik D. 2018)
Figure 1 DNA Vaccines Induce Adaptive Immune Responses (Hobernik D. 2018)
Routes of DNA Vaccine Delivery
To transfect as many APCs as possible, targeting DNA vaccine delivery to secondary lymphoid organs via a systemic application like intravenous injection, or oral or pulmonary administration is a suitable strategy. Alternatively, DNA vaccines are applied often topically via the skin or intramuscularly. Intravenous application of DNA vaccines in principle allows reaching APC located in secondary lymphoid organs. However, the physicochemical characteristics of DNA-complexing Nano-carriers (NC) offer the advantage to shield the DNA vaccine from degradation by DNases and other enzymes. NC determines the biodistribution of the DNA vaccine. In general, numerous types of NC were demonstrated to interact with blood components which thereby form a protein corona around the NC which in turn may strongly affect its cellular binding properties. For oral administration of DNA, non-pathogenic bacterial strains are often used as vectors, termed bacto-fection. In the intestine, these bacteria may be phagocytosed directly by mucosal DC/macrophages spreading extensions into the gut lumen or after M cell-mediated transcytosis at the Peyer's patches. After phagocytosis, plasmid DNA is released from the phagolysosome, and the numerous bacteria-associated danger signals result in profound APC activation. Aside from the oral application, bacteria were shown to confer transfection of APC when applied at other mucosal sites. More recently, the DNA vaccine delivery properties of orally applied bacteria, for example concerning phagolysosomal escape have been improved by additional coating with NC. Compared with other systemic routes of DNA vaccination, the pulmonary application of aerosolized DNA vaccines is a rather new approach. The usage of naked and NC-complexed DNA was demonstrated to yield transfection of lung epithelial cells. Hence, so far research focuses on the treatment of local gene defects as in the case of cystic fibrosis. The skin constitutes an interesting target organ for DNA vaccination due to the rather high frequency of cutaneous DC. Different transdermal DNA vaccination strategies have been developed, and their suitability is clinically tested.
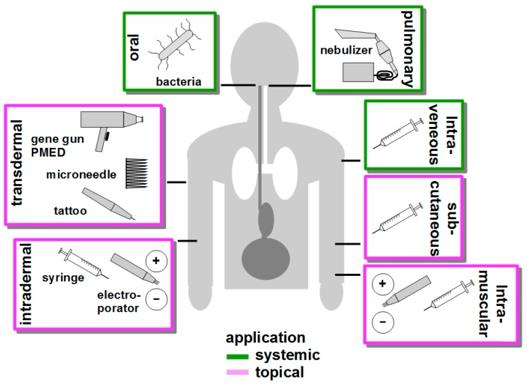 Figure 2 Routes of DNA Vaccine Delivery (Hobernik D. 2018).
Figure 2 Routes of DNA Vaccine Delivery (Hobernik D. 2018).
Concerning the distribution of DNA vaccines complexed with NC, it is noteworthy that small particles are easily transported into the lymph node, while larger particles remain longer at the site of administration. About the distribution of DNA vaccines complexed with NC, it is noteworthy that small particles are easily transported into the lymph node, while larger particles remain longer at the site of administration. After subcutaneous injection small PEGylated liposomes were found in a larger amount in the lymph node than after intravenous or intraperitoneal injection.
Optimization of DNA Vaccines
DNA vaccines still face many challenges to become an effective tool as their success achieved in preclinical studies has not been translated into the clinic yet. The biggest challenge is the low immunogenicity of DNA vaccines in bigger animals and humans probably due to the difficulty to upscale the DNA vaccine amounts used in small animal systems. For this, about 5-20 mg of DNA would have to be injected into an average-sized human. If naked or unformulated plasmid DNA is used for vaccination, an important factor that contributes to low therapeutic efficiency is DNA degradation. Studies showed that plasmids, compared to other administration forms like minicircles, are degraded relatively fast within one week. DNA molecules that successfully enter the cell need to pass the barrier of the nuclear membrane to be transcribed. DNA molecules that successfully enter the cell need to pass the barrier of the nuclear membrane to be transcribed.
Important optimization steps that improve DNA transfection efficiency comprise the introduction of DNA-complexing nano-carriers aimed to prevent extracellular DNA degradation, enabling APC targeting, and enhancing endo/lysosomal escape of DNA. Attachment of virus-derived nuclear localization sequences facilitates nuclear entry of DNA. Improvements in DNA vaccine design include the use of APC-specific promotors for transcriptional targeting, the arrangement of multiple antigen sequences, the co-delivery of molecular adjuvants to prevent tolerance induction, and strategies to circumvent potential inhibitory effects of the vector backbone. Successful clinical use of DNA vaccines may require combined employment of all of these parameters, and combination treatment with additional drugs.
DNA Vaccines in the Clinic
In the US, at the moment over 500 clinical trials that focus on DNA vaccination are registered, targeting especially viral infections and cancer, while bacterial infections and autoimmune diseases are less of a topic. The use of DNA vaccines has early raised safety concerns mainly concerning the probability of stable integration of transfected DNA into the genome of somatic or even germ cells, causing dysregulated gene expression and mutations. Concerning unwanted DNA vaccination-associated immune effects, repeated intramuscular application of a luciferase-encoding reporter vector in primates resulted in long-term reporter expression but induced no anti-DNA antibodies. The potential transfer of prokaryotic elements of DNA vaccines like antibiotic resistance genes into eg, the gut microbiome has been considered another issue of safety concerns, but so far, no such event has been documented. Nonetheless, the aforementioned as well as additional safety concerns of DNA vaccines need to be considered regarding their translational use in the clinic. Presently, there are no approved DNA vaccines for use in humans. Nevertheless, some DNA-based vaccines were approved by the FDA and the USDA for veterinary use, including a vaccine against West Nile Virus in horses and canine melanoma. One of the first human clinical trials with DNA vaccines evaluated the therapeutic and prophylactic effects against HIV infection in which no significant immune responses but potential immunogenicity was detected. The overall safety of DNA vaccines has been thoroughly proven in several clinical trials, underlined by the fact that no antibody response against prokaryotic parts of the DNA vaccine itself has been observed and that adverse effects are limited to mild local reactivity at the injection site. Moreover, clinical trials employing DNA vaccines evoked efficient induction of cellular and humoral responses. However, the level of these responses most often was not sufficient to elicit significant clinical benefits. Therefore, numerous clinical trials focus on DNA vaccine optimization strategies to augment their immunogenicity. In addition, it is necessary to compare the suitability of different DNA vaccine delivery routes to yield potent adaptive immune responses.
Reference
- Hobernik D, Bros M. DNA Vaccines-How Far From Clinical Use? Int J Mol Sci. 2018 Nov 15;19(11):3605. Distributed under Open Access license CC BY 4.0, without modification.
