Modes of Delivery and Intracellular Synthesis of Small Regulatory RNAs
An Overview
Small regulatory RNAs are non-coding RNA (ncRNA) molecules, such as microRNAs (miRNAs), piwi-interacting RNAs (piRNAs), small nucleolar RNAs (snoRNA), small nuclear RNAs (snRNAs), and small interfering RNAs (siRNAs), which play a role in cellular processes and turn the expression of mRNAs on and off in response to environmental signals, such as activation or inhibition processes. These RNAs function to regulate gene expression via numerous mechanisms. The mechanisms include binding to protein targets, protein modification, binding to mRNA targets, and regulating gene expression.
Intracellular Synthesis of Small Regulatory RNAs
miRNAs are one of the most studied RNAs among the different categories of small ncRNAs. miRNAs are transcribed by RNA polymerase II to form primary miRNAs (pri-miRNAs). These pri-miRNAs are then processed in the nucleus by a complex DiGeorge syndrome critical region 8 (Drosha-DGCR8) to form precursor miRNAs (pre-miRNAs) that are exported to the cytoplasm by the exportin 5 system. Within the cytoplasm, they are further processed by the Dicer-TRBP (TAR RNA-binding protein 2) complex to generate the 18-22-nt double-strand mature miRNA.
- piRNAs
piRNAs range between 26 and 31 nucleotides long. They are usually intragenic or organized in clusters. They are transcribed as single-strand precursor RNAs from genomic regions containing transposable elements and repetitive sequences. They mature in the cytoplasm and associate with PIWI proteins, a subfamily of Argonaute proteins including PIWIL1/HIWI, PIWIL2/HILI, PIWIL3, and PIWIL4/HIWI2.
- snoRNAs
snoRNAs range between 60 and 300 nucleotides long and are located in the nucleolus or in Cajal bodies. They are divided into two categories, ones containing boxes C and D; and, others containing boxes H and ACA. snoRNAs are generated after splicing, debranching, and trimming of mRNA introns. Then, mature snoRNAs associate with proteins to produce small nucleolar ribonucleoproteins (snoRNPs). These complexes can be exported into the nucleolus to participate in rRNA processing.
- snRNAs
snRNAs are almost 150-nt-long ncRNAs. snRNAs are transcribed by RNA polymerase II or III before maturation in the nucleus. They are components of the spliceosome where they participate in pre-mRNA processing.
siRNAs are a class of double-stranded RNA and also belong to non-coding RNA molecules, 20-25 base pairs in length. They are from cytoplasmic long double-stranded RNAs (dsRNAs) that are cleaved in the cytoplasm by Dicer into functional siRNAs. They are recognized by the Ago2/RISC (Argonaute 2 containing RNA-induced silencing complex (RISC)) complex that interacts to degrade one strand. Another strand leads the inhibitory complex to perfect or closely perfect complementary mRNA. Targeted mRNAs or ncRNAs are subsequently degraded through cleavage by the RISC complex.
Modes of Delivery of Small Regulatory RNAs
Recently, there is tremendous progress in delivering small regulatory RNAs using various biocompatible biodegradable, and nontoxic biopolymers. They can be divided into three types-synthetic polymers, lipid and lipid-like materials, and conjugating a bioactive ligand to RNA.
Synthetic polymers, like poly-L-lysine, polyamidoamine, and polyethyleneimine, as well as naturally occurring polymers like chitosan, have all been used for delivering RNA, with varying levels of success. In addition, poly (β-amino esters) have also proved to be capable of effecting the delivery of siRNA.
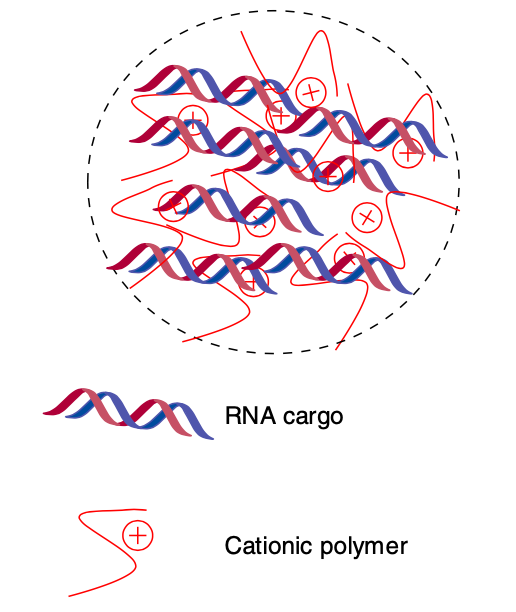 Figure 1. Polymeric nanoparticles comprising RNA and cationic polymer. (Kaczmarek, 2017)
Figure 1. Polymeric nanoparticles comprising RNA and cationic polymer. (Kaczmarek, 2017)
- Lipid and lipid-like materials
Various lipid-based vesicles like microemulsions, liposomes and lipid nanoparticles have been investigated for the targeted delivery of ncRNAs. Liposomes are neutral or cationic amphiphilic lamellar lipoidal vesicular structures including a hydrophilic 'head' (a neutral or positively charged polar group) and a hydrophobic 'tail' (non-polar fatty acid or cholesterol). RNA molecules can be entrapped within the neutral vesicle and form stable nucleic acid lipid particle which offers prolonged circulation and enhanced accumulation at the vascular leakages. In terms of lipid nanoparticles (LNPs) by the addition of other hydrophobic moieties, such as cholesterol and PEG-lipid, in addition to an ionizable/cationic lipid, LNPs enhance nanoparticle stability and can significantly increase the efficacy of RNA delivery.
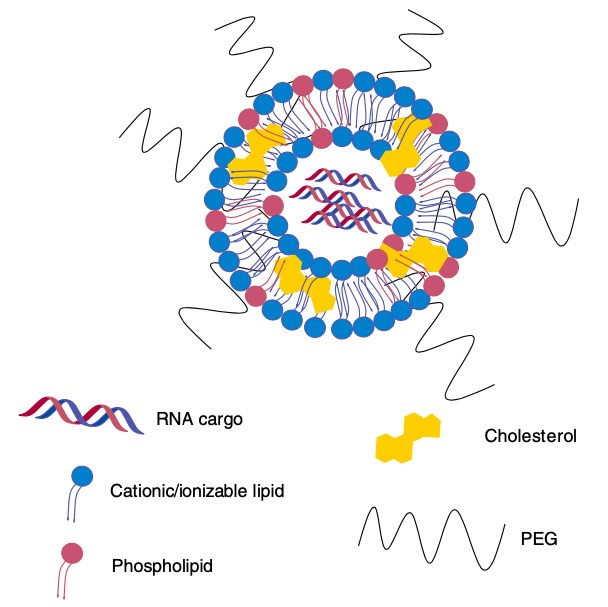 Figure 2. Lipid nanoparticles containing RNA. (Kaczmarek, 2017)
Figure 2. Lipid nanoparticles containing RNA. (Kaczmarek, 2017)
As an alternative to nanoparticles, a more conceptually straightforward and chemically well-defined means of delivery is to directly conjugate a bioactive ligand to the RNA that will allow it to enter the cell of interest. For example, the conjugation of N-acetylgalactosamine (GalNAc), which targets the asialoglycoprotein receptor on hepatocytes, to siRNA. GalNAc conjugates are able to rapidly enter systemic circulation and target the liver. Besides that, some researches showed that cholesterol, vitamin E, antibodies, and cell-penetrating peptides are also available as bioactive ligands conjugated to RNA.
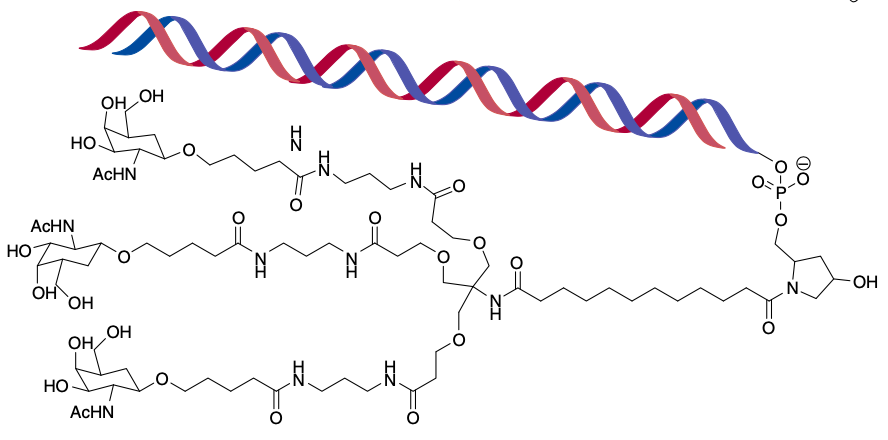 Figure 3. Chemical structure of a tertiary conjugate between GalNAc and RNA. (Kaczmarek, 2017)
Figure 3. Chemical structure of a tertiary conjugate between GalNAc and RNA. (Kaczmarek, 2017)
Reference
- Kaczmarek, J. C.; et al. (2017). Advances in the delivery of RNA therapeutics: from concept to clinical reality. Genome medicine. 9(1), 60. Distributed under Open Access license CC BY 4.0, without modification.
