Antisense Oligonucleotide (ASO) In Vitro Screening Service
Antisense oligonucleotides (ASOs) are crucial research and therapeutic tools, offering strong support for investigating gene function, disease mechanisms, and the development of novel therapies. After ASO sequence design, synthesis, and production, a carefully designed set of in vitro experiments is required to validate the activity and function of the designed ASO, including the detection of mRNA-level inhibition efficiency and assessment of protein expression levels. Creative Biolabs offers professional ASO in vitro screening services, providing clients with efficient, accurate, and customized experimental solutions.
Antisense Oligonucleotide in Drug Development
The therapeutic potential of antisense oligonucleotides (ASOs) stems from their ability to precisely control gene expression—an advantage of traditional small molecule drugs over primary protein-targeting drugs. ASOs are being developed to treat a variety of diseases, especially those with a clear genetic basis.
| Mechanism of Action | Molecular Outcome | Therapeutic Application |
|---|---|---|
| RNase H-Mediated Degradation | Cleavage and degradation of target mRNA. | Gene knockdown to treat diseases caused by overexpressed or toxic proteins. |
| Splice Modulation (Exon Skipping/Inclusion) | Steric hindrance to alter pre-mRNA splicing, leading to a functional/restored protein. | Treatment of genetic diseases where a mutation causes an incorrect splice product. |
| Steric Hindrance | Blocking ribosomal assembly or function, preventing translation without mRNA degradation. | Inhibition of non-coding RNA function or modulation of translation. |
Antisense Oligonucleotide (ASO) In Vitro Screening Introduction
Antisense oligonucleotides (ASOs) are short-chain synthetic single-stranded oligonucleotides, typically 15 to 30 nucleotides in length, designed to bind to specific target RNA sequences via Watson-Crick base pairing. The in vitro screening phase is a critical stage during which a large number of synthetic ASO candidates are evaluated to determine their efficacy, specificity, and cell tolerance before conducting costly in vivo studies. This process is essential for:
- Validate Target Engagement: Confirming that the ASO binds to the target mRNA or precursor mRNA.
- Assess Potency: Determining the minimum effective concentration required to achieve the desired biological effect.
- Evaluate Mechanism of Action (MOA): Validating whether the ASO functions through its intended pathway (e.g., RNase H cleavage, splicing regulation, or steric hindrance).
- Identify Off-Target Effects: Screening for unexpected binding to non-target RNAs, which is crucial for predicting safety and specificity.
A Flexible Suite of Services at Creative Biolabs
ASO Design and Optimization
We utilize proprietary algorithms and extensive historical data to design optimized ASO libraries for your target genes. Our design platform integrates multiple parameters, including GC content, self-complementarity, cross-species homology, and SNP factors, to maximize success rates. We offer design services for all major antisense oligonucleotide (ASO) mechanisms, including interstitial oligonucleotides, splice-conversion oligonucleotides, and sterically hindered oligonucleotides, and are compatible with various chemical modifications.
ASO Efficacy Screening
We utilize relevant cell models for multiple efficacy assessments and employ appropriate endpoints based on the mechanism of action:
- mRNA quantification via RT-qPCR and digital PCR
- Protein analysis via Western blotting, ELISA, and flow cytometry
- Splicing analysis via RT-PCR and capillary electrophoresis
- Functional assessment via pathway-specific reporter gene detection and phenotypic readings
- Our efficacy screening platform has medium-throughput capabilities, allowing parallel evaluation of dozens to hundreds of ASO sequences, and employs rigorous standardization methods to control for variations in delivery efficiency.
Detection of Gene Silencing Efficiency
- RT-PCR
RNA is extracted from ASO-treated cells or tissues, and the expression level of target RNA is determined using techniques such as real-time reverse transcription-PCR (qRT-PCR) to evaluate the gene-silencing effect of ASO.
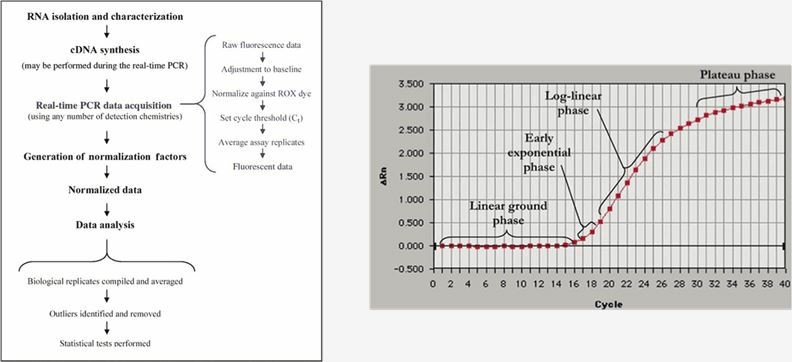 Fig.1 Schematic diagram of RT-PCR procedure and analysis of amplification curve.1
Fig.1 Schematic diagram of RT-PCR procedure and analysis of amplification curve.1
- Western Blotting
Western Blotting can be used to detect the expression level of target proteins. By comparing the protein expression levels before and after ASO treatment, the knockout efficiency of ASO can be evaluated.
- Flow Cytometry
Flow cytometry can detect the expression level of specific proteins in cells or on the cell surface, evaluate the knockout effect of ASO, and analyze the correlation between various indicators.
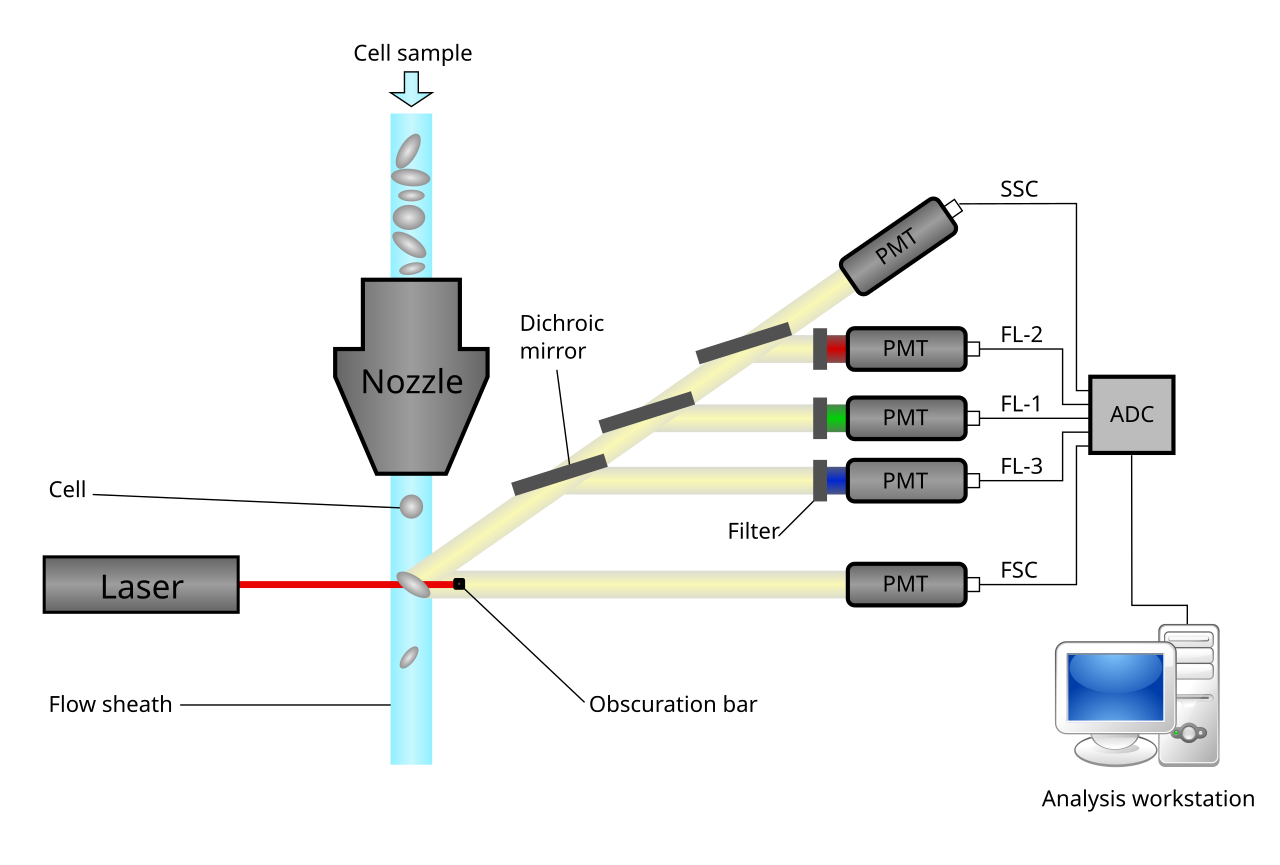 Fig.2 Working principle of flow cytometer.Distributed under Open Access license CC BY 4.0, from Wiki without modification.
Fig.2 Working principle of flow cytometer.Distributed under Open Access license CC BY 4.0, from Wiki without modification.
- Cell Function Experiment
According to the function of the silenced target genes, cell function tests such as MTT, CCK8, CTG, cell invasion, and scratch tests can be performed for cell cycle, cell proliferation, apoptosis, and cell killing efficacy.
- Reporter gene assay
A reporter gene system containing a target RNA sequence was constructed to evaluate the knockout efficiency of ASO.
-
Analysis of detection results
- Efficient silencing: If the experimental results show that the expression levels of target mRNA and target protein are significantly reduced, and an ASO is evenly distributed in the cell, then the ASO can be considered to have an efficient silencing effect.
- Specificity: By comparing the silencing effect of different sequences of oligonucleotides on target genes, the specificity can be assessed.
- Mechanism of action: Based on the distribution of oligonucleotides in the cell, the mechanism of action can be reasonably speculated.
Our Collaboration Process
We collaborate with clients through a streamlined, milestone-driven process:
-
Phase 1
Exploratory Consultation - We begin with comprehensive consultation to gain a deep understanding of your treatment goals, target biology, and specific challenges, thus initiating all collaboration. This phase includes detailed discussions of mechanism of action preferences, routes of administration, and development timelines.
-
Phase 2
Project Design - Based on the exploratory consultation, we develop a customized screening protocol, outlining the model system, detection methods, success criteria, and project timeline. This protocol includes a detailed cost structure and identifies key decision points throughout the process.
-
Phase 3
Model System Validation - Before initiating full screening, we establish and validate a biologically meaningful detection system, including confirming target expression, functional testing, and determining an appropriate screening window.
-
Phase 4
Iterative Screening Execution - We implement a stratified screening strategy, regularly updating progress and sharing preliminary data at predetermined milestones. Our team provides interim reports, detailing results to date and providing recommendations for subsequent screening phases based on emerging data patterns.
-
Phase 5
Lead Compound Characterization - For selected lead compounds, we conduct more in-depth characterization, including dose-response relationships, time-course analysis, and preliminary mechanism of action studies.
-
Phase 6
Technology Transfer - Upon completion of the screening process, we will provide a complete data package and methodological details to support a smooth transition to downstream development activities. For ongoing collaborations, we will establish a continuous support mechanism to further optimize or expand the screening process.
Platform Advantages and Highlights
High-fidelity in vitro screening platform: From two-dimensional to three-dimensional. The in vivo efficacy of ASO drugs is closely related to the physiological relevance of in vitro screening models. Creative Biolabs' platform emphasizes the use of high-fidelity models to eliminate the predictive bias inherent in traditional immortalized cell lines.
A. Selection and Optimization of Disease-Relevant Cell Models
| Model Type | Advantage | Key Screening Focus |
|---|---|---|
| iPSC-Derived Cells | High physiological relevance, patient-specific genetics; suitable for complex tissues like neurons and cardiomyocytes. | Toxicity Prediction, Functional Rescue. |
| Primary Cells | Closest to in vivo physiological state. | Early Specificity Validation. |
| Organoids (3D Culture) | Mimic 3D tissue structure and cell interactions, simulating tissue penetration. | Tissue Targeting, Efficacy Prediction. |
B. Other Cell Lines:
| Species | Disease | Cell line |
|---|---|---|
| Human | Non-tumor cell line | HEK293T, HUVECS |
| Hepatocellular cancer cell line | HepG2, Huh-7, Hep3B | |
| Lung cancer cell line | A549, H1975, H358 | |
| Hematologic tumor cell line | THP1, Jurkat, SUP-T1, NALM-6 | |
| Cervical cancer cell line | Hela | |
| Murine | —— | L929S, CHO, Raw264, 4T1, B16-F10 |
C. Organoids: Organoids grown in a three-dimensional environment more closely resemble the organization and physiology of natural epithelial cells than 2D cultured cells. ASO can be taken up efficiently by organoids without transfection reagents, making it a convenient tool for routine laboratory research and screening.
D. High-Throughput Screening (HTS) Technology Integration
High-throughput screening (HTS) is crucial for handling the vast ASO sequence library:
- Automation and Miniaturization: Utilizing 384-well or 1536-well plates, combined with automated liquid handling robots, thousands of ASO candidate sequences can be processed rapidly and in parallel. This significantly shortens the screening cycle and improves efficiency.
- Data Quality Control: Strict application of statistical indicators such as the Z' factor ensures the stability and reliability of the screening data, forming the foundation of the high-fidelity HTS platform.
Frequently Asked Questions
Q: What is the general timeline for a full ASO screening campaign?
A: Our standard screening process usually takes about 8-12 weeks from sequence design to primary compound screening (if needed) and is based on the screening depth and complexity of the model system used. A rapid screening campaign can be achieved by starting with a predefined ASO library, in which case preliminary activity data can be generated in as little as 4 weeks. A more extensive screening process that includes delivery optimization and safety profiling will usually take about 14-16 weeks. For our personalized ASO screening using patient-derived organoids we have developed an optimized and expedited process that provides functional data within 6-8 weeks of receipt of patient samples.
Q: Can you screen a wide variety of chemical modifications and ASO designs?
A: We can screen all major ASO chemical modifications currently available including phosphate thioester backbones, 2'-O-methyl (2'-OMe), 2'-O-methoxyethyl (2'-MOE), locked nucleic acids (LNA), restricted ethyl (cEt), phosphoryldiamine morpholino oligomers (PMO), and peptide-conjugated PMOs (PPMO). We are particularly experienced with 2'-OMePS ASO and its conjugation to cell penetrating peptides through click chemistry.
Q: Delivery of ASOs is often a critical challenge during screening. How do you approach this?
A: We utilize a range of parallel delivery approaches, including electroporation, lipid nanoparticles, and novel peptide conjugation to ensure robust delivery across a variety of models. We systematically optimize the delivery conditions for each cell type as we have found large differences in delivery efficacy for different biological models. We have also developed specialized delivery approaches for the most challenging primary cells and organoids to maximize ASO activity while also preserving cell health.
Q: How do you know in vitro models will be predictive of in vivo efficacy?
A: We have performed extensive correlation studies across a range of programs comparing in vitro screen results and subsequent in vivo performance and found strong evidence to support the predictive power of our stratified screen approach, particularly when advanced model systems like 3D organoids and primary cells are used. If your candidate drugs are screened with afterglow nanoparticles for biodistribution, it can also ensure that candidate drugs selected for in vivo testing have good biodistribution characteristics which is also good at predicting transformation.
Connect with Us Anytime!
The convergence of ASO chemistry, delivery technologies, and advanced cell models has created unprecedented opportunities for the development of transformative oligonucleotide therapies. Creative Biolabs' platform remains at the forefront of these developments, continuously integrating technological innovations such as afterglow imaging and rapid organoid generation to improve screening efficiency and predictive capabilities. As the ASO field continues to evolve towards more complex targets and personalized applications, our flexible screening methods are constantly being adapted to address these emerging challenges. Contact us today for a quotation or any question. Our customer service representatives are available 24 hours a day!
Reference
- Wong, Marisa L., and Juan F. Medrano. "Real-time PCR for mRNA quantitation." Biotechniques 39.1 (2005): 75-85. 10.2144/05391RV01 (Distributed under Open Access license CC BY 4.0, without modification.)
