Hydrodynamic Intravascular Injection
The development of an efficient and safe gene delivery method is indispensable for molecular biology research and the progress in the following gene therapy. Recently, hydrodynamic gene delivery (HGD) using a rapid injection of a relatively large volume of the solution has opened up a new avenue for both basic research and gene therapy studies. This method is superior to the existing delivery systems due to its high efficiency, simplicity, and low risk in triggering immune responses and carcinogenesis. Currently, DNA, RNA, proteins and synthetic compounds have been successfully delivered to cells in various tissues of small animals through the hydrodynamic principles.
The Basic of HGD
HGD is a method that facilitates the delivery and expression of genetic material in target cells through an intravenous injection. It is developed based on advances in our understanding of the structure and properties of blood capillaries, and the dynamic properties of fluids passing through blood vasculature. The basic principle is that a large volume of solution, containing genes or oligonucleotides, RNA, proteins or other compounds, is infused rapidly in vasculatures to facilitate substance transfer into parenchyma cells. The original method of HGD in small animals involves tail vein injection of plasmid DNA solution in 5-7 s in a volume equal to 8%-10% of the body weight. It showed a fairly high level of transgene expression in various organs, with gene expression in the liver being the highest. The mechanism of HGD is shown in Fig.1. The injected plasmid solution directly enters the heart, causing cardiac congestion and backflow to the inferior vena cava and hepatic veins. This solution then reaches the sinusoids; the hydrodynamic pressure expands the liver, enlarges the fenestrae of the endothelium, and forces invagination of the cell membrane of the hepatocytes to allow the DNA to move into the cytoplasm.
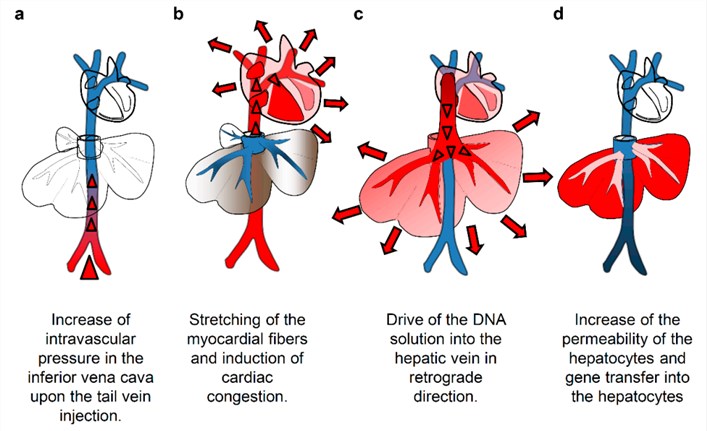 Figure 1. Mechanism of hydrodynamics-based delivery to the mouse liver. (Kamimura, 2015)
Figure 1. Mechanism of hydrodynamics-based delivery to the mouse liver. (Kamimura, 2015)
HGD to Various Tissues
Although most research has focused on tail vein injections using rodents as animal models, HGD has also been explored for gene delivery into various tissues and animal species. Table 1 summarizes cases where successful HGD has been achieved in the liver, kidney, skeletal muscle, myocardium carotid artery, etc. These experiments also demonstrate the safety of the HGD and the possibility of repeated treatments. For its application in humans, the most advanced hydrodynamic program is the delivery of hydrodynamic genes to muscles.
Table.1 Tissues and animal species in which hydrodynamic delivery has been reported. (Suda, 2007)
HGD in General Research
HGD has been widely used for basic and translational research. It is used as a research tool for introducing macromolecules into cells to study their biochemical, cell biological physiological, or/and therapeutic activity in the context of a whole animal, including functional analysis of regulatory elements, gene function analysis, delivery of viral vector, establishing disease animal model, genetic immunization, expression of the therapeutic gene, etc (Table 2). The most popular application of HGD is in gene therapy studies. Examples include expression of the gene for possible treatment of growth hormone deficiency, phenylketonuria diabetes, obesity, myocarditis, liver regeneration, glomerulonephritis, xenotransplantation and various types of cancer.
Table.2 Summary of applications of hydrodynamic delivery in basic and translational research. (Suda, 2007)
HGD in Clinical Application
The high efficiency and simplicity of HGD have raised interest among the gene therapy community toward its possible applications in humans. The main focus has centered on the reduction of the injection volume and the development of a procedure that would create the necessary hydrodynamic pressure for gene transfer. A common strategy employed to reduce injection volume is to deliver the hydrodynamic genes directly to the vasculature of target tissues. For example, Zhang et al have shown that the volume required for successful HGD can be reduced to 3 ml in a 200-250 g rat by targeting an isolated liver through the hepatic vein. Recently, Yoshino et al have shown that hydrodynamic injection of 100-150 ml of DNA solution into the hepatic or portal vein of pigs is safe. These results indicate that it is clinically acceptable to transfer liver genes to hepatocytes in a 50 kg man at a volume of 500-750 ml.
The other focus on HGD is the development of an effective and safe method for delivering a pressurized injection that will also be efficient in gene delivery. This will require a specially designed injection device that can push a volume of hundreds of milliliters in a few seconds. An ideal device would be a computer-assisted system enabling real-time control of the injection based on the hydrodynamic pressure at the injection site of the tissue. Precise control of injection can avoid tissue damage caused by too heavy injection or low gene delivery efficiency and eliminate the need for consideration of individual variability in body weight and vascular structure, which further promotes the clinical application of HGD.
Reference
- Kamimura, K.; et al. (2015). Image-Guided Hydrodynamic Gene Delivery: Current Status and Future Directions. Pharmaceutics. 7(3):213-223. Distributed under Open Access license CC BY 4.0, without modification.
