Overview of Magnetofection-Based Gene Delivery
Magnetofection is a transfection method that uses a magnetic field to guide the complex formed by nucleic acid molecules and magnetic carriers to target tissues or cells. It can be divided into three types: in vitro, in vivo and in situ. It has the characteristics of high transfection efficiency, good biocompatibility, and a controllable transfection process, aiming to realize different types of gene therapy, such as gene replacement, gene knockout, gene silencing, gene editing, etc. The principle of magnetofection is to use the interaction between the complex and the magnetic field to increase the contact opportunity between the complex and the cell surface, promote the endocytosis of the complex, and release nucleic acid molecules into the cytoplasm or nucleus through different mechanisms to achieve gene expression or regulation. Magnetofection is of great significance in the treatment of diseases, because it can be used to carry different types of nucleic acid molecules to treat a variety of diseases, such as tumors, genetic diseases, infectious diseases, etc.
Components, Types, and Operation Steps of Magnetofection
The main components required for magnetofection include magnetic carriers, nucleic acid molecules, and magnetic fields. Magnetic carrier refers to nanoparticles with superparamagnetism, usually composed of iron oxide (Fe3O4 or γ-Fe2O3) or other metal oxides (such as MnFe2O4 or CoFe2O4), and whose surfaces are modified with polymers or other functional molecules to improve their biocompatibility, stability, and targeting. The magnetic carrier can be prepared by methods such as the co-precipitation method, the high-temperature thermal decomposition method, the hydrothermal method, or the microemulsion method. Nucleic acid molecules refer to exogenous genes with therapeutic effects, which can be naked DNA, plasmid DNA, RNA, or other nucleic acid analogs, and form complexes with magnetic carriers through electrostatic interactions or covalent bonds. The term magnetic field refers to an external stable or gradiently changing strong magnetic field, which can be a permanent magnet or an electromagnet, and is used to guide the complex to the target tissue or target cell.
There are three different types of magnetofection: in vitro magnetofection, in vivo magnetofection, and in situ magnetofection. In vitro magnetofection refers to transfecting the cultured cells with the complex in vitro, and then returning the transfected cells to the body. This type of magnetofection is suitable for pretreatment or expansion of cells, such as stem cell gene therapy. In vivo magnetofection refers to injecting the complex directly into the body and concentrating it on the target tissue or target cells by a magnetic field. Such magnetofection is suitable for gene therapy for local tissues, such as tumor gene therapy. In situ magnetofection refers to injecting the complex directly into the target tissue or target cells and holding it in place with a localized magnetic field. It is suitable for situations where gene therapy is performed on tissues or cells that are difficult to target, such as gene therapy for the nervous system.
There are five main steps in magnetofection. First, prepare the complex: mix the nucleic acid molecule and the magnetic carrier in a certain ratio and incubate in an appropriate buffer for a period of time to form a stable complex. Second, transfect cells: add the complex to cultured cells and incubate for a period of time under appropriate conditions to make contact with cells. Third, apply a magnetic field: place the culture dish under a strong magnetic field and incubate under appropriate conditions for a period of time so that the complex is adsorbed to the cell surface or enters the cell. Fourth, remove the magnetic field: remove the culture dish from the strong magnetic field and replace it with fresh culture medium so that the transfected cells can return to their normal growth state. Fifth, detect the transfection effect: according to the type and function of the nucleic acid molecule, select an appropriate method to detect the existence, expression, and effect of the nucleic acid molecule in the transfected cells.
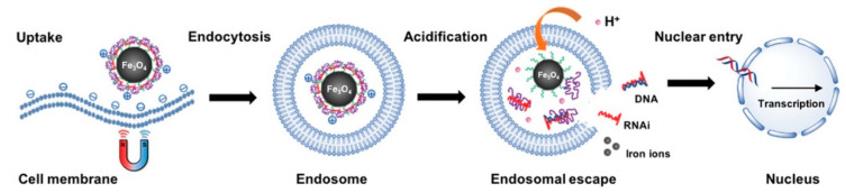 Figure 1. Magnetofection Using Magnetic Nanoparticles.1
Figure 1. Magnetofection Using Magnetic Nanoparticles.1
The Mechanism of Magnetofection
The mechanism of magnetofection mainly includes the following aspects: how magnetofection promotes the transfection efficiency and stability of nucleic acid molecules; how magnetofection affects the processes of endocytosis, nuclear localization, and gene expression.
The action of the magnetic field makes the complex form high-density magnetic spots on the target tissue or target cells, which increases the chance of contact between the complex and the cell surface, thereby improving the transfection efficiency. At the same time, the magnetic field can also protect the complex from degradation or clearance in the blood circulation, increasing the half-life of the complex in vivo, thereby improving the transfection stability. In addition, the magnetic field can also achieve precise control of the transfection process and transfection location, avoiding the toxicity and immune response of the complex to non-target tissues or cells, thereby improving the safety of transfection.
The size, shape, surface modification, and magnetic properties of a magnetic carrier all affect its interaction with the cell membrane, thereby affecting the manner and efficiency of its endocytosis. Generally speaking, spherical magnetic carriers smaller than 200 nm mainly enter cells through receptor-mediated endocytosis, while non-spherical magnetic carriers larger than 200 nm mainly enter cells through phagocytosis. Different types of endocytosis lead to complexes entering different types of endosomes, for example, vesicular endocytosis leads to complexes entering early endosomes and late endosomes, Phagocytosis causes the complex to enter the phagosome. These endosomes are likely to fuse with lysosomes, causing the complex to be degraded. Therefore, to increase transfection efficiency, it is necessary to facilitate the escape of the complex from the endosome into the cytoplasm.
In order to achieve the purpose of gene therapy, nucleic acid molecules need to enter the nucleus from the cytoplasm and be transcribed and expressed in the nucleus. However, nucleic acid molecules usually cannot directly enter the nucleus through the nuclear pore and need to rely on some auxiliary factors or signal sequences. A commonly used method is to use a nuclear localization signal (NLS), that is, a cationic amino acid-rich peptide segment, combined with nucleic acid molecules or magnetic carriers, enabling it to interact with receptors on nuclear pores and be thereby transported into the nucleus. Another commonly used method is to use the cytoskeletal network, such as microtubules and microfilaments, to transport the complex along the gradient to the region close to the nuclear membrane, thereby increasing its probability of entering the nucleus. In addition, there are some other methods, such as using electroporation, ultrasound, laser, and other physical means to open the nuclear membrane; using chemical means such as lysozyme, triglyceride lipase, and low pH buffer to degrade the nuclear membrane; using DNA binding proteins, histone deacetylase inhibitors, DNA methylation inhibitors, and other biological means to regulate gene expression.
Clinical Progress of Magnetofection
Magnetofection can be used to carry different types of nucleic acid molecules, such as DNA, RNA or other nucleic acid analogs, to achieve different types of gene therapy, such as gene replacement therapy, gene knockout therapy, gene silencing therapy, gene editing therapy. Magnetofection has demonstrated its superior transfection effect and therapeutic effect in a variety of disease models, such as tumors, genetic diseases, and infectious diseases. For example, in melanoma cells, magnetofection can achieve efficient p53 gene transfection and induce apoptosis. In vascular endothelial cells, magnetofection can achieve high-efficiency VEGF gene transfection and promote angiogenesis. In HCC cells, magnetofection can achieve efficient miR-122 gene silencing and inhibit HCC growth. Magnetofection enables efficient CRISPR/Cas9 gene editing and repair of Duchenne muscular dystrophy mutations in mouse muscle.
At present, magnetofection is still in the pre-clinical stage, and formal clinical trials have not yet been conducted. However, some preliminary clinical observations and evaluations have shown the safety, feasibility, and effectiveness of magnetofection in humans. For example, in an exploratory clinical observation of patients with advanced melanoma, the use of magnetofection to transfect the IL-12 gene into the subcutaneous tumors of patients showed good local tolerance and an anti-tumor immune response. As another example, in an exploratory clinical evaluation of patients with acute myocardial infarction, the use of magnetofection to transfect the VEGF gene into the patient's myocardial tissue showed good cardiac function improvement and angiogenesis. In addition, in an exploratory clinical evaluation of hemophilia B patients using magnetofection to transfect the FIX gene into the patient's liver tissue, the results showed good FIX expression levels and coagulation function.
Despite the many advantages of magnetofection, there are some challenges that need to be overcome before it can be extended to clinical applications. The first question is how to improve the targeting and selectivity of the complex in vivo. At present, magnetofection mainly relies on an external magnetic field to achieve the targeting and selectivity of the complex in vivo, but this method has certain limitations, such as complex operation, low precision, and a small range. In order to solve this problem, some ligands or antibodies with specific recognition ability can be considered to modify the complex so that it can bind to specific receptors or markers on target tissues or cells, thereby improving its targeting and selectivity. The second question is how to improve the biostability and biocompatibility of the complex in vivo. At present, the complexes used by magnetofection may be degraded or aggregated by the in vivo environment, thereby reducing its transfection efficiency and safety. In order to solve this problem, some biostable and biocompatible materials can be considered to prepare or encapsulate the complex, so that it can resist changes in the in vivo environment and reduce toxicity and immunity to non-target tissues or non-target cells. reaction.
Reference
- Sizikov AA, Nikitin PI, Nikitin MP. Magnetofection In Vivo by Nanomagnetic Carriers Systemically Administered into the Bloodstream. Pharmaceutics. 2021 Nov 14;13(11):1927. Distributed under Open Access license CC BY 4.0, without modification.
