The human intestine's complex structure and its various cellular functions play essential roles in taking up nutrients, regulating immune responses, and maintaining interactions between the host and microbes. Impaired functioning of this essential organ leads to numerous debilitating disorders including inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), and colorectal cancer. Intestinal organoids have opened up a groundbreaking period for research in gastrointestinal science. The remarkable 3D constructs known as intestinal organoids originate from adult intestinal stem cells (ISCs) or pluripotent stem cells (PSCs) and demonstrate the exceptional ability to self-organize into structures that replicate the in vivo intestinal epithelium with crypt-villus-like domains and differentiated cell types including enterocytes and goblet cells alongside Paneth and enteroendocrine cells. Their physiological relevance turns intestinal organoids into essential models for research on intestinal development as well as its normal functioning and disease states.
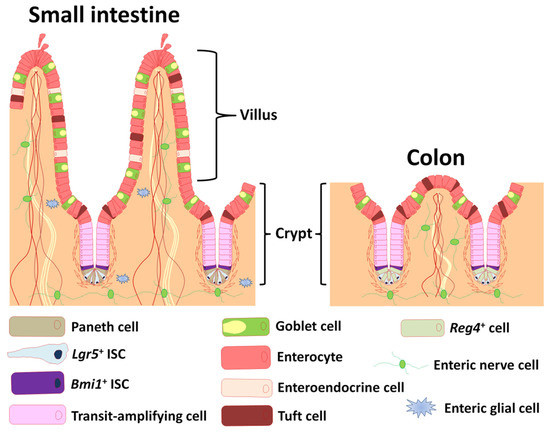 Figure 1 Depiction of the anatomy of intestinal epithelium and enteric nervous system.1,3
Figure 1 Depiction of the anatomy of intestinal epithelium and enteric nervous system.1,3
What Are Intestinal Organoids?
Intestinal organoids serve as miniature 3D structures which self-assemble at a microscopic level to accurately duplicate the essential features of native intestinal tissue within an experimental environment. Intestinal organoids advance beyond traditional cell cultures because they serve as more realistic models for gut biology research.
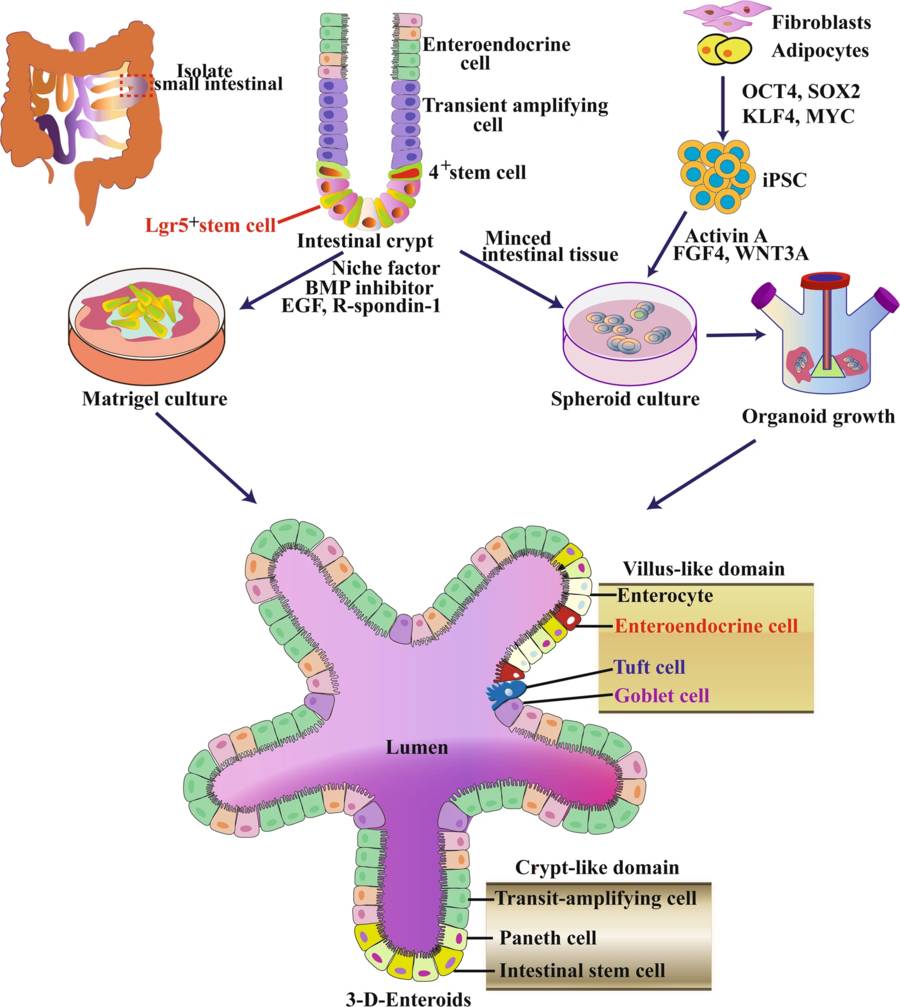 Figure 2 Schematic diagram of intestinal tissue engineering by organoid technology.2,3
Figure 2 Schematic diagram of intestinal tissue engineering by organoid technology.2,3
Intestinal Organoids Source
The main sources of intestinal organoids consist of PSCs and ISCs. Researchers initially produced crypt-villus intestinal organoids named the 'ENR system' through the cultivation of mouse intestinal tissue in a designated specialized medium. Subsequent work demonstrated that adding recombinant Wnt3A to the ENR medium enabled the cultivation of mouse colon organoids, leading to further optimization for human small intestine and colon organoid culture. This groundbreaking research created a new scientific domain that specializes in 3D in vitro culture systems with a focus on ISCs. Intestinal organoids now replicate some aspects of native intestinal tissue structure and function which enables new paths for research and treatment of intestinal diseases.
Characteristics of Intestinal Organoids
-
3D Architecture: Organoids develop spherical structures with an internal lumen and an inward-facing apical surface which resembles the intestinal lumen found in living organisms as opposed to flat 2D cultures.
-
Cellular Heterogeneity: The diverse collection of epithelial cell types present in the native intestine exists within intestinal organoids alongside multipotent intestinal stem cells and their differentiated descendants.
-
Self-Organization and Self-Renewal: Organoid stem cells utilize internal developmental pathways to both renew themselves and transform into different types of intestinal cells which enables the maintenance of its structure throughout long-term culture periods.
-
Physiological Functionality: The organoids possess native intestinal functional characteristics through their ability to form barriers, secrete mucus, and express essential transporters and digestive enzymes.
How to Culture Intestinal Organoids?
The typical culture process for intestinal organoids includes the isolation of intestinal crypts that contain Lgr5+ adult intestinal stem cells or the differentiation of pluripotent stem cells into intestinal progenitors. The cells undergo embedding in a specialized extracellular matrix such as Matrigel followed by culturing in a growth medium with essential factors. The essential components of organoid culture typically feature Wnt activators (such as Wnt3a for human and colon organoids), R-spondin which acts as a Wnt agonist, epidermal growth factor (EGF), and Noggin which functions as a BMP inhibitor. The general workflow involves:
01 Tissue Dissociation
The isolation of crypts or single cells from biopsied or resected intestinal tissue requires enzymatic or mechanical tissue dissociation.
02 ECM Embedding
The isolated cells/crypts get immersed in a liquid ECM solution which is then dispensed into droplets that form solid gels.
03 Culture Medium Supplementation
Overlaying the ECM domes with a specialized organoid growth medium.
04 Incubation
Culturing at 37°C with 5% CO2, allowing for self-organization and growth.
05 Passaging
Regularly disrupting and splitting the growing organoids to maintain long-term cultures and expand their numbers.
Intestinal Organoids on a Chip
The combination of intestinal organoids with microfluidic "organ-on-a-chip" (OOC) technology marks a major advancement. Through this inventive method researchers are empowered to build more dynamic and physiologically accurate microenvironments for organoid culture. By embedding organoids within microfluidic devices, researchers can:
-
Mimic Mechanical Forces: Introduce fluid flow and mechanical stretch, similar to the peristalsis of the gut, which can influence organoid development and function.
-
Control Nutrient/Waste Gradients: Establish precise gradients of nutrients, oxygen, and signaling molecules, more accurately reflecting in vivo conditions.
-
Co-culture with Other Cell Types: Integrate immune cells, endothelial cells, or nerve cells to create more complex multicellular systems that better mimic the intestinal microenvironment.
-
Enable Real-Time Monitoring: Facilitate real-time observation and analysis of organoid responses to various stimuli.
Applications of Intestinal Organoids
Disease Modeling
Researchers need to construct effective in vitro models for multiple intestinal diseases including IBD types like Crohn's disease and ulcerative colitis, celiac disease and infectious diseases such as viral bacterial and parasitic infections alongside inherited intestinal disorders like cystic fibrosis and short bowel syndrome.
Intestinal organoids serve as effective platforms for conducting high-throughput drug candidate screenings. The three-dimensional structure combined with physiological relevance found in intestinal organoids enables more precise forecasting of drug absorption and effectiveness relative to traditional two-dimensional cell lines.
Tissue Engineering
The potential of intestinal organoids to create transplantable functional intestinal tissue remains a focus of current research. Researchers aim to develop complex tissue grafts for repairing severely damaged or diseased bowel segments through a combination of organoids with biofabrication strategies and scaffolds.
Developmental Biology
Researchers study intestinal development alongside morphogenesis and cell differentiation using intestinal organoids as a precise in vitro model. This helps researchers understand how the complex architecture of the intestine is formed and maintained.
Comparison of Intestinal Organoids and Traditional Models
Intestinal organoids represent advanced three-dimensional cell culture systems that replicate the structure and function of the native intestine. Traditional models such as 2D cell cultures and animal models fall short compared to intestinal organoids which demonstrate superior performance in mimicking human physiology and disease conditions. Despite their benefits intestinal organoids possess specific drawbacks.
|
Feature/Model
|
2D Cell Cultures
|
Intestinal Organoids
|
Animal Models
|
|
Physiological Relevance
|
Limited; lack 3D architecture, cell-cell/matrix interactions, and tissue-specific microenvironment. May lose original cell characteristics over time.
|
High; mimic tissue architecture, cellular diversity, and physiological functions (e.g., crypt-villus structure, epithelial cell types).
|
Very High; represent the complete organism, including complex interactions between different organs, systems, and the immune system.
|
|
Complexity
|
Low; typically single cell type, simple growth on a flat surface.
|
Moderate; multiple cell types, self-organization into 3D structures. Can be co-cultured with other cell types (e.g., mesenchymal, immune cells).
|
High; intact physiological systems, including vasculature, nervous system, immune system, and microbiome.
|
|
Cost
|
Low
|
Moderate
|
High
|
|
Genetic Stability
|
Low; prone to genomic changes with passaging.
|
High; retain genetic and phenotypic traits of source tissue.
|
High; stable genome over generations (within species).
|
|
Heterogeneity
|
Low; often clonal or limited diversity.
|
High; can retain intra-tissue heterogeneity (e.g., patient-derived organoids).
|
High; represent the genetic and phenotypic diversity of a population.
|
|
Access/Manipulation
|
Easy; direct access to cells for observation, gene editing, and functional assays.
|
Challenging for apical lumen access (can be overcome with techniques like "inside-out" organoids or microfluidics). Easier for genetic manipulation than animals.
|
Challenging; requires specialized techniques for in vivo manipulation and observation. Ethical considerations.
|
|
Drug Screening/Toxicity
|
Limited predictivity for in vivo response; may miss off-target effects or complex drug interactions.
|
Improved predictivity compared to 2D; can assess efficacy and toxicity in a more tissue-relevant context.
|
Gold standard for preclinical drug testing, but still has limitations in translating to human response.
|
|
Disease Modeling
|
Limited; may not accurately recapitulate complex disease mechanisms.
|
Good for modeling human diseases, especially those involving epithelial function and stem cell biology (e.g., IBD, cancer, infectious diseases).
|
Excellent for modeling systemic diseases and complex interactions.
|
Frequently Asked Questions
Q: What is the difference between an organoid and a spheroid?
A: Organoids represent a more advanced form of 3D cell culture than spheroids due to their complexity. Organoids originate from stem cells or primary tissue and include multiple cell types which self-organize into complex structures that resemble organ-specific features while they are frequently cultured in an ECM. Spheroids originate from immortalized cell lines and typically contain only one cell type within their simpler structure and are usually cultured as freely floating aggregates.
Q: How long can intestinal organoids be expanded in culture?
A: Organoid lines can be expanded through numerous passages (exceeding 25 passages in certain instances) and sustained over months to years when stem cell populations receive uninterrupted support and correct passaging methodologies are applied. Extended passages could result in minor changes to cell characteristics.
Q: Can intestinal organoids be cryopreserved?
A: The process of cryopreservation enables intestinal organoids to be stored long-term for convenient experimental use. To enhance cell survivability following thawing researchers typically suggest pre-treating with a ROCK inhibitor.
Elevate Your Research with Creative Biolabs' Advanced Intestinal Organoids Models
As Creative Biolabs continues to push the boundaries of this technology, integrating advanced bioengineering, multi-cellular co-culture, and microfluidic platforms, the future promises even more sophisticated and predictive models. These advancements will not only deepen our fundamental knowledge of the intestine but also accelerate the development of groundbreaking therapies and personalized treatments for the millions affected by gastrointestinal disorders worldwide.
Organoid Models
Organoids Related Products
Creative Biolabs delivers advanced intestinal organoid models through custom generation and intricate culture methods along with detailed characterization and functional testing. Partner with us to use intestinal organoids to propel your biomedical research which leads to better human health outcomes. Contact us today to learn more!
References
-
Takahashi T, Fujishima K, Kengaku M. Modeling intestinal stem cell function with organoids. International journal of molecular sciences, 2021, 22(20): 10912.https://doi.org/10.3390/ijms222010912
-
Tian C, Yang M, Xu H, et al. Stem cell-derived intestinal organoids: a novel modality for IBD. Cell Death Discovery, 2023, 9(1): 255. https://doi.org/10.1038/s41420-023-01556-1
-
Distributed under Open Access license CC BY 4.0, without modification.
Research Model
Related Sections:

 Figure 1 Depiction of the anatomy of intestinal epithelium and enteric nervous system.1,3
Figure 1 Depiction of the anatomy of intestinal epithelium and enteric nervous system.1,3
 Figure 2 Schematic diagram of intestinal tissue engineering by organoid technology.2,3
Figure 2 Schematic diagram of intestinal tissue engineering by organoid technology.2,3