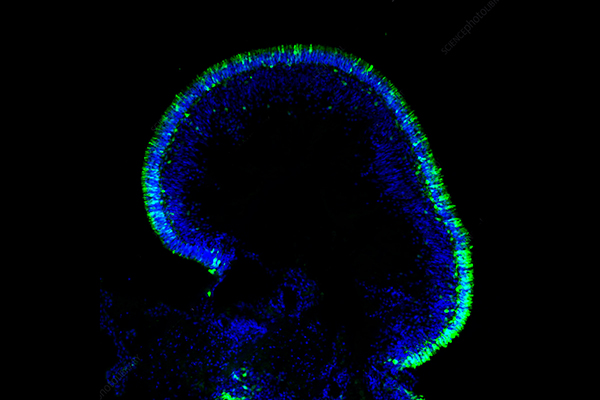
Pancreatic Organoids Introduction
The pancreas, a vital organ nestled behind the stomach, plays a dual role in our body: producing digestive enzymes and secreting crucial hormones like insulin and glucagon. Dysfunction in this organ can lead to devastating diseases, most notably diabetes and pancreatic cancer, one of the deadliest forms of cancer. For decades, scientists have grappled with the challenges of studying pancreatic diseases, often relying on traditional 2D cell cultures or animal models that fail to fully mimic the complexity of human biology. However, the emergence of the pancreatic organoid model offers a novel solution. Pancreatic organoids accurately replicate the microenvironment of the pancreas, allowing for a more precise reflection of pancreatic cell functions and interactions. This advancement not only enhances our understanding of pancreatic physiology and pathology but also brings new hope for the treatment of diseases like diabetes and pancreatic cancer.
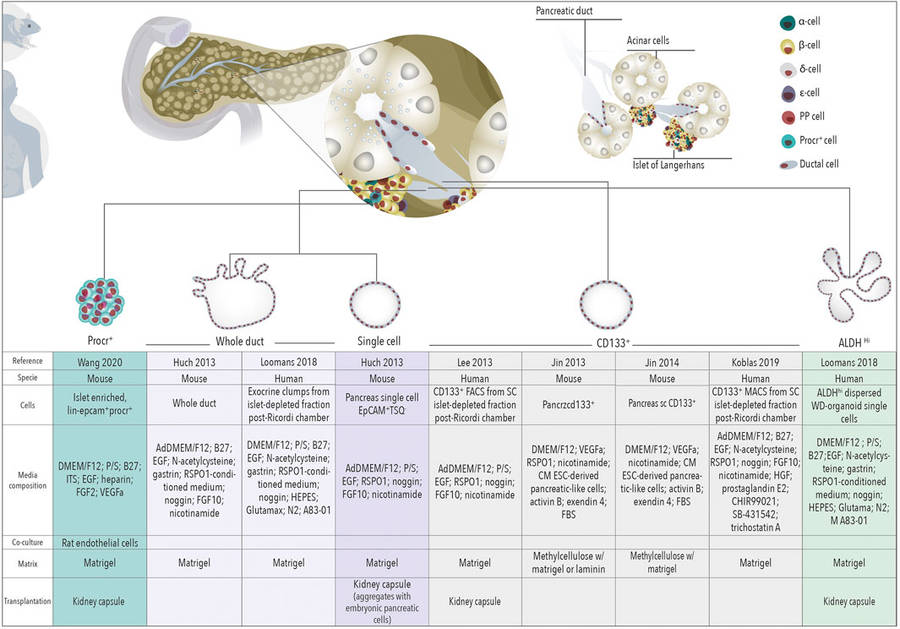 Figure 1 Schematic representation of organoids derived from different cells of the healthy pancreas.1,3
Figure 1 Schematic representation of organoids derived from different cells of the healthy pancreas.1,3
What are Pancreatic Organoids?
Organoids are essentially simplified, scaled-down versions of organs, derived from stem cells or primary tissue, that can recapitulate key aspects of the original organ's architecture, cell composition, and function. Pancreatic organoids are miniature, three-dimensional (3D) cellular structures grown in the lab that mimic the key functional, structural, and biological complexity of a real pancreas. They are derived from stem cells (either adult stem cells or induced pluripotent stem cells) or directly from pancreatic tissue (healthy or diseased, such as tumor tissue).
Sources of Pancreatic Organoids
Pancreatic organoids can be generated from various sources, each offering unique advantages for specific research questions:
Adult Stem Cells (ASCs)
ASCs are a foundational source for generating pancreatic organoids, offering models that closely mirror the physiological and pathological conditions of the human pancreas. This approach leverages the inherent self-renewal and differentiation capabilities of resident stem or progenitor cells within adult pancreatic tissue.
Induced Pluripotent Stem Cells (iPSCs)
These iPSCs have the remarkable ability to differentiate into any cell type in the body. By carefully directing their differentiation with specific growth factors and signaling molecules, scientists can coax them into forming pancreatic organoids that mimic fetal pancreas development, often containing all three major pancreatic cell lineages.
Patient-Derived Tissue (PDOs)
This is particularly crucial for pancreatic cancer research. Tumor biopsies or surgical resections from patients can be used to grow patient-derived organoids (PDOs). These PDOs are invaluable because they retain the genetic and molecular characteristics, as well as the heterogeneity, of the original patient's tumor.
Fetal Tissue
The experimental investigation revealed that embryonic mouse pancreatic progenitor cells can be cultured to generate self-organized 3D structures. These structures exhibit robust tri-lineage differentiation into acinar, ductal, and endocrine cell types.
How to culture Pancreatic Organoids?
Pancreatic organoids are miniature 3D structures grown in the lab that mimic the complexity of the real pancreas. They are powerful tools for studying pancreatic diseases.
01 Tissue Procurement and Dissociation
-
Isolate cells from pancreatic tissue (either healthy or tumor).
-
Use enzymes (e.g., collagenase, dispase) and mechanical methods to break down the tissue into single cells or small cell clusters.
02 Extracellular Matrix (ECM) Embedding
-
Mix the dissociated cells with cold Matrigel (or a similar basement membrane extract). Matrigel provides the 3D scaffold for the cells.
-
Dispense small droplets of the cell-Matrigel mixture into culture plates.
-
Incubate the plate upside down at 37°C to allow the Matrigel domes to solidify.
03 Specialized Growth Medium
-
Carefully add a specialized growth medium containing a precise cocktail of growth factors and signaling molecules on top of the solidified Matrigel domes. These components are crucial for cell proliferation and self-organization.
-
Key components often include: R-Spondin1 (a Wnt pathway agonist), EGF, FGF10, Noggin, A-83-01, and ROCK inhibitor (Y-27632) (especially for the initial few days to improve cell survival).
04 Culture and Maintenance
-
Place the plates in a humidified incubator.
-
Change the medium every 2-3 days and regularly observe organoid growth and morphology under a microscope.
05 Passaging
-
When organoids grow too large or become too dense, they need to be passaged.
-
Mechanically break the organoids into smaller fragments or dissociate them into single cells (e.g., by pipetting vigorously).
-
Re-embed these fragments or cells into fresh Matrigel and add new growth medium.
Advantages and Limitation of Pancreatic Organoids
 Advantages of Pancreatic Organoids
Advantages of Pancreatic Organoids
The successful development of pancreatic organoids marks a significant leap forward in modeling complex organs. Unlike traditional 2D cell cultures, organoids accurately replicate the 3D architecture, cell-cell interactions, and functional attributes of native organs. Human fetal pancreatic organoids (hfPOs), in particular, are unparalleled in their ability to generate acinar, ductal, and endocrine cell types, a feat rarely achieved by other model systems. This technology holds immense promise for disease modeling. Current research into pancreatic diseases like diabetes, chronic pancreatitis, and pancreatic cancer often relies on animal models or single cell lines, which fall short in capturing the intricate complexity of the human pancreas. Pancreatic cancer organoids offer significant advantages including:
-
High Fidelity
-
Rapid Generation
-
Versatile Applications
-
Functional Insights
 Limitation of Pancreatic Organoids
Limitation of Pancreatic Organoids
While human pancreatic organoids offer significant functional and cellular diversity, the technology faces inherent limitations. Primarily, current culture methods rely on exogenous matrices like BME or Matrigel, whose variable composition and source hinder standardization for clinical applications. Future advancements will therefore prioritize developing synthetic, well-defined matrix materials. Additionally, hfPOs inadequately mimic the complex tissue microenvironment due to their lack of non-epithelial components such as blood vessels, immune cells, and neural tissue. The pancreas's functional integrity relies on the synergy between diverse cell types; for instance, blood vessels supply essential nutrients and oxygen to endocrine cells, and neurons regulate hormone secretion. Consequently, integrating organoids with microfluidic chip technology to create a multi-cellular "pancreas-on-a-chip" represents a crucial future direction for organoid research.
Applications of Pancreatic Organoids
Beyond pancreatic cancer, organoids are broadly applicable in various researches:
 Figure 2 Application of patient-derived pancreatic cancer organoid.2,3
Figure 2 Application of patient-derived pancreatic cancer organoid.2,3
Developmental Biology
Organoids derived from ESCs or iPSCs are excellent tools for studying the intricate processes of pancreatic development, including cell differentiation and tissue formation. This can provide insights into congenital pancreatic disorders.
Diabetes Research
Pancreatic organoids, particularly those with a focus on endocrine cell differentiation, are being used to study beta cell function, insulin production, and the mechanisms underlying diabetes. The long-term goal is to potentially generate functional beta cells for transplantation therapies.
Organoids can serve as a platform to assess the safety and toxicity of new drugs, providing an early indication of potential adverse effects on pancreatic cells.
Regenerative Medicine
While still in its early stages, the ability to generate functional pancreatic tissue in vitro holds promise for future regenerative therapies, potentially offering a source of cells for transplantation in patients with pancreatic damage or diabetes.
Key Technical Approaches for Pancreatic Cancer Organoid Research
Pancreatic cancer organoids are sophisticated in vitro 3D tumor models, derived from patient tissues or induced pluripotent stem cells (iPSCs), engineered to accurately mimic the biological complexities of in vivo tumors. Current leading technical approaches include:
-
Patient-Derived Organoids (PDOs): This process involves extracting tumor cells from surgical or biopsy samples, followed by enzymatic digestion and 3D culture in a matrix gel (e.g., Matrigel) supplemented with growth factors (e.g., EGF, Wnt).
-
Gene-Edited Organoids (GEOs): Utilizing advanced gene editing tools like CRISPR-Cas9, common pancreatic cancer driver mutations (e.g., KRAS, TP53, CDKN2A) are introduced into normal pancreatic organoids.
-
Multi-Omics Integration and Organoid-on-a-Chip Technology: This cutting-edge approach combines single-cell sequencing, spatial transcriptomics, and microfluidic organ-on-a-chip platforms to enhance organoid fidelity and high-throughput analytical capabilities.
-
Dynamic Drug Sensitivity Detection via Chip Technology: This principle involves continuous drug perfusion through microfluidic channels, coupled with real-time monitoring of organoid responses using fluorescent markers (e.g., Calcein-AM/PI for live/dead cells) or metabolic indicators (e.g., glucose/lactate sensors).
-
Tumor Microenvironment (TME) Simulation and Immune Interaction Analysis on Chip: This technique implements co-culture systems within a chip, integrating pancreatic cancer organoids with various cell types like fibroblasts, immune cells (e.g., T cells, macrophages), or vascular endothelial cells.
-
High-Throughput Phenotypic Screening Chip Technology:
-
Microwell Array Chip: Organoids are distributed into thousands of microwells, enabling automated microscopy for high-throughput screening of phenotypes (e.g., invasion, apoptosis).
-
Droplet Microfluidics: Individual organoids are encapsulated in droplets containing fluorescent reporter genes, allowing for single-clone level analysis.
Frequently Asked Questions
Q: What are pancreatic organoids?
A: Pancreatic organoids are miniature, self-organizing 3D structures grown in the lab that mimic key aspects of the human pancreas's architecture, cell composition, and function. They are derived from stem cells (adult, embryonic, or induced pluripotent) or directly from patient tissues.
Q: How are organoids different from traditional 2D cell cultures or spheroids?
A: 2D Cell Cultures: Cells grow in a single flat layer on a plastic surface, losing their natural 3D organization and many physiological interactions.
Spheroids: These are 3D aggregates of cells, but they often lack the complex cellular heterogeneity, self-organization, and distinct tissue-like structures seen in organoids. Organoids, by definition, exhibit a higher degree of self-organization and often require an extracellular matrix to grow.
Q: Why are pancreatic organoids so important for research?
A: They provide a more physiologically relevant model than traditional methods, allowing scientists to:
-
Study disease mechanisms (e.g., cancer progression, diabetes development) more accurately.
-
Test drugs more effectively, including personalized drug screening for cancer patients.
-
Investigate human pancreatic development.
-
Potentially develop regenerative therapies.
Conclusion
Pancreatic organoids represent a transformative technology with immense potential to accelerate biomedical research and clinical translation. They offer a unique, human-relevant platform that bridges the gap between traditional 2D cell cultures and complex animal models. Despite the significant challenges in achieving full functional maturity, complex architecture, and scalability, the rapid pace of innovation in stem cell biology, tissue engineering, and biofabrication is continuously pushing the boundaries. These mini-pancreases are already providing unprecedented insights into the mechanisms of diabetes, guiding the development of new drugs, and offering a personalized approach to understanding and treating pancreatic diseases.
Elevate Your Research with Creative Biolabs' Advanced Pancreatic Organoids Models
Creative Biolabs leverages cutting-edge technology and expertise to develop and provide high-quality pancreatic organoids tailored to research, drug discovery, and personalized medicine needs. Our services empower scientists and clinicians to unravel pancreatic biology, model disease pathogenesis, and expedite the development of novel treatments with unprecedented precision and relevancy.
Organoid Models
Organoids Related Products
Creative Biolabs is proud to be at the forefront of this exciting revolution. We are committed to leveraging the power of pancreatic organoids to translate groundbreaking research into tangible solutions for patients. Partner with us to unlock the full potential of these mini-pancreases and drive forward the future of pancreatic health. Contact us today to learn more!
References
-
Casamitjana J, Espinet E, Rovira M. Pancreatic organoids for regenerative medicine and cancer research. Frontiers in Cell and Developmental Biology, 2022, 10: 886153. https://doi.org/10.3389/fcell.2022.886153
-
Bengtsson A, Andersson R, Rahm J, et al. Organoid technology for personalized pancreatic cancer therapy[J]. Cellular Oncology, 2021, 44: 251-260. https://doi.org/10.1007/s13402-021-00585-1
-
Distributed under Open Access license CC BY 4.0, without modification.
Research Model
Related Sections:

 Figure 1 Schematic representation of organoids derived from different cells of the healthy pancreas.1,3
Figure 1 Schematic representation of organoids derived from different cells of the healthy pancreas.1,3
 Figure 2 Application of patient-derived pancreatic cancer organoid.2,3
Figure 2 Application of patient-derived pancreatic cancer organoid.2,3