It has been proven that antibody therapy is highly powerful for cancer treatment. Antibody-dependent cell-mediated cytotoxicity (ADCC) and complement dependent cytotoxicity (CDC) are two important cell killing mechanisms of antibody drugs. Besides, for certain therapeutic bispecific IgGs and Fc-containing bispecific antibodies (BsAbs), ADCC is very important for their in vivo efficacy. To test if therapeutic BsAb candidates can efficiently induce ADCC, various ADCC assays are employed. Creative Biolabs provides various ADCC assays for the functional analysis of bispecific antibodies. Abiding the strict quality standards, Creative Biolabs assures the efficacy profiles of the therapeutic antibodies for customers.
ADCC is a type of cell-mediated immune defenses and is part of the adaptive immune response. During ADCC, specific antibodies (e.g., IgG) bind membrane surface antigens on target cells with the Fab domains and effector cells with the Fc region, which leads to the active lysis of the target cells mediated by the effector cells. Effector cells in a classical ADCC process are normally natural killer (NK) cells, although macrophages, neutrophils, and eosinophils are also seen to mediate ADCC. NK cells express Fc receptors (mostly CD16 or FcγRIII) on the cell surface. These receptors recognize and bind to the Fc region of an antibody and then cytokines such as IFN-γ are released to kill tumor cells. Eosinophils express FcεRI on the cell surface, which binds to IgE Fc portion. IgE can coat large parasites in the circulation. Then the interaction between eosinophils and parasite-bound IgEs generates a signal for the eosinophils to degranulate.
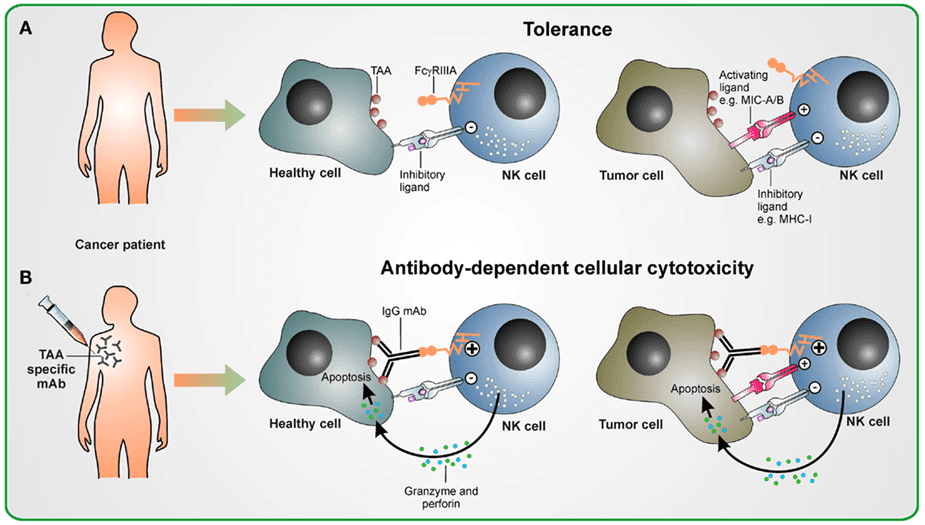
Figure 1. Schematic overview of the antibody-dependent cellular cytotoxicity (ADCC) in therapeutic antibody treatment (Seidel, U. J., 2013).
Peripheral blood mononuclear cells (PBMCs) and NK cells are two types of effector cells used in ADCC tests, while the 51Cr approach and LDH approach are widely adopted to measure the extent of cell lysis. All these methods are available in Creative Biolabs for customers to choose from. The readout for these assays is target cell lysis endpoint and thus provides accurate measurements.
The 51Cr Approach
Prior to the assay, isotope 51Cr is pre-loaded into the target cells. In the ADCC assay, target cells lysed by the ADCC process will release 51Cr into the system. After separating the supernatant from the rest components, radioactivity in the supernatant can be quantified. The extent of cell lysis can be calculated by comparing the supernatant radioactivity to the original load.
The LDH Approach
The lactate dehydrogenase (LDH) approach is another simple and fast ADCC assay. It quantifies the release of LDH, which is a stable cytoplasmic enzyme present in all cells, from cells lysed via the ADCC process. LDH is rapidly released into the supernatant upon damage of the cell membrane and the LDH activity can be determined by measuring enzymatic reactions such as formazan formation.
Creative Biolabs provides not only top BsAb production services but also superior BsAb analysis services. Creative Biolabs is pleased to assist you with our advanced antibody engineering and analysis platforms. Our high quality ADCC assay will greatly contribute to the success of your projects. Creative Biolabs also provides other various services regarding BsAbs development. Please feel free to contact us for more information and a detailed quote.
Reference
-
Seidel, Ursula JE, Patrick Schlegel, and Peter Lang. "Natural killer cell mediated antibody-dependent cellular cytotoxicity in tumor immunotherapy with therapeutic antibodies." Frontiers in immunology 4 (2013): 76. (Under open access license CC BY 3.0, without modification.)
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY





