Structure Hot Targets Published Data
Immune redirection is not limited to T lymphocytes; natural killer (NK) cells, key cytotoxic elements of innate immunity, eliminate tumor and virus‐infected cells. Their lytic granules, containing perforin and granzyme, mirror those of cytotoxic T cells but generally prompt less cytokine release. Thus, targeting NK cells is an appealing anti‐tumor strategy.
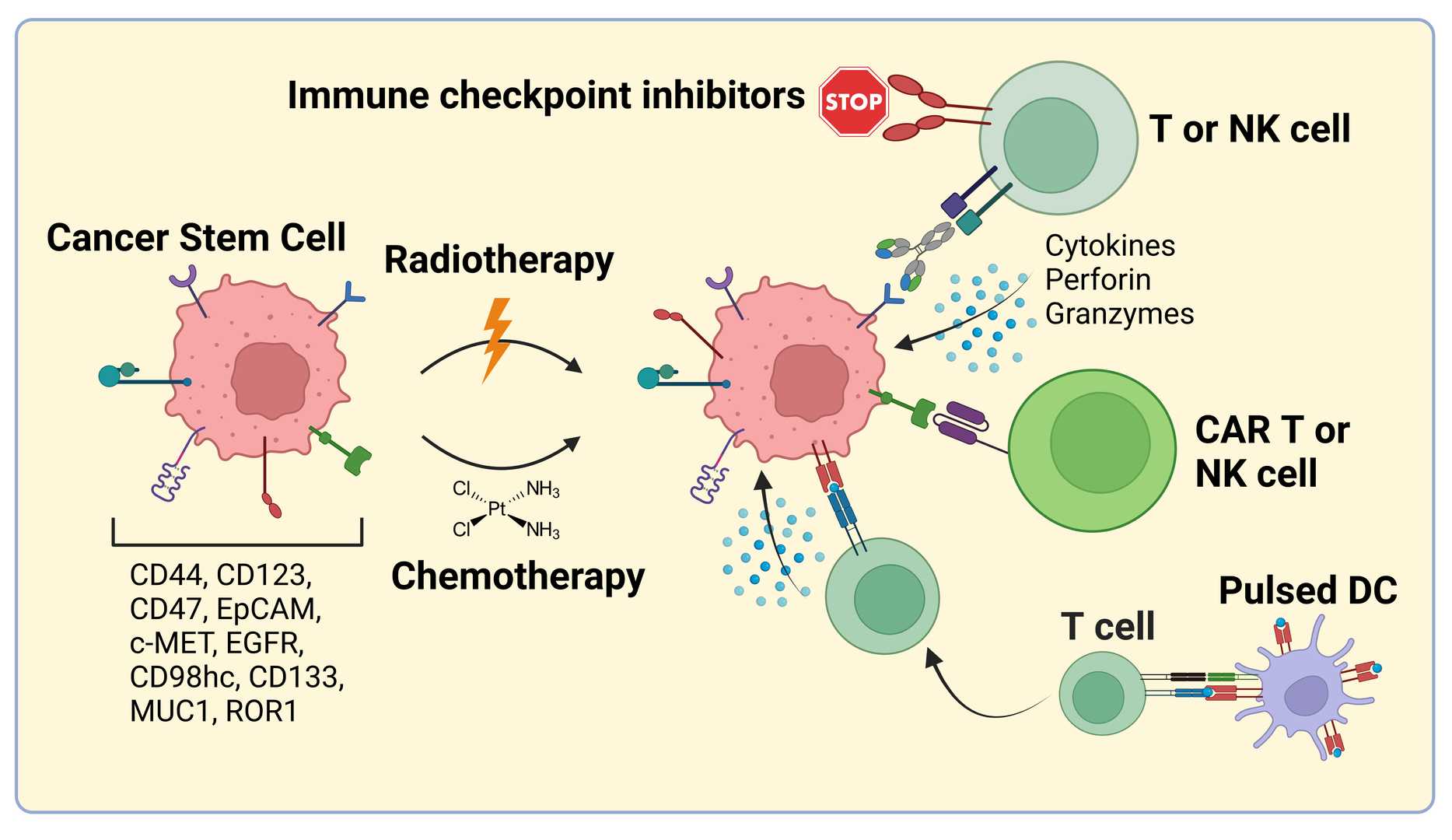 Fig.1 Schematic depiction of immunotherapeutic strategies targeting cancer stem cells, featuring bispecific antibodies and antibody–drug candidates.1
Fig.1 Schematic depiction of immunotherapeutic strategies targeting cancer stem cells, featuring bispecific antibodies and antibody–drug candidates.1
Creative Biolabs offers engineered bispecific antibody platforms and NK Cell Engager (NKCE) Based BsAb Design Service that address diverse clinical needs while circumventing drug resistance. Bispecific NKCE based antibodies and trispecific NKCE based antibodies enable direct targeting of tumors, thereby reducing resistance and severe side effects. These innovative approaches hold significant promise for improved therapeutic outcomes.
Structures of NKCE-Based BsAbs Design
NK cells are cytotoxic constituents of the innate immune system, crucial in tumor immunosurveillance. Creative Biolabs offers approaches to harnessing NK cells against cancer that employs tandem single-chain variable fragments, as seen in bispecific or trispecific NKCE based-antibodies. These constructs simultaneously target CD16A on NK cells and one or two tumor-associated antigens present on various solid tumors. Alternatively, two scFvs can be conjoined via a modified human IL-15 crosslinker to yield TriKEs, which bolster NK cell persistence and activation in vivo.
Our NKCE based BsAbs can be broadly divided into two formats based on their architecture:
-
IgG-like BsAbs retain an Fc region, granting them Fc-mediated effector capabilities like antibody-dependent cellular cytotoxicity (ADCC). Their incorporation of the constant region facilitates antibody purification, enhances solubility and stability, and extends their circulating half-life in vivo.
-
Non-IgG-like BsAbs: are characterized by a considerably smaller molecular weight (approximately 55–60 kDa), which reduces immunogenicity and improves safety. Their compact size enhances tumor tissue penetration; however, this advantage comes at the cost of a shorter half-life.
Hot Targets in NKCE-Based BsAbs Design
-
Hematological malignancies: CD19 & CD16 BsAbs have been shown to augment NK cell-mediated cytotoxicity in vitro, whereas CD16 & CD33 have effectively redirected NK cells toward myelodysplastic syndrome (MDS).
-
Solid tumor: Strategies include EGFR & CD16A BsAbs; CD16 & EpCAM BsAbs; CD16 & EpCAM & IL-15
Published Data
NKCEs constitute a groundbreaking class of antibody-based treatments within the field of cancer immunotherapy. A series of recent investigations examined NKCEs utilizing single-domain antibodies (sdAbs) targeting NKp30 to direct NK cells toward tumor cells expressing the epidermal growth factor receptor (EGFR). The findings revealed that NKCEs, which bivalently target both EGFR and engage NKp30, surpass their monovalent counterparts in enhancing NK cell-mediated lysis of tumor cells. This underscores the capacity of NKCEs to amplify the cytotoxic efficacy of NK cells against malignancies. By leveraging dual-targeting mechanisms, these NKCEs offer a promising strategy to potentiate the immune system's natural ability to combat cancer, thereby highlighting their potential as a powerful tool in the development of next-generation cancer therapies. This research provides critical insights into optimizing NK cell engagement for therapeutic purposes, marking a significant advancement in immunotherapeutic approaches to treating cancer.
Creative Biolabs maintains an adept research team with expertise in BsAbs, empowered by state-of-the-art engineering platforms. We deliver bespoke NKCE-based BsAbs development services that span design, engineering, production, and exhaustive analysis.
Reference
-
Köseer, Ayse Sedef, et al. "Immunotargeting of cancer stem cells." Cancers 15.5 (2023): 1608. Distributed under Open Access license CC BY 4.0, without modification.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY


 Fig.1 Schematic depiction of immunotherapeutic strategies targeting cancer stem cells, featuring bispecific antibodies and antibody–drug candidates.1
Fig.1 Schematic depiction of immunotherapeutic strategies targeting cancer stem cells, featuring bispecific antibodies and antibody–drug candidates.1


